Abstract
Rationale. Obstructive sleep apnoea (OSA) is common in coronary artery disease (CAD) and a possible cause of increased mortality. To date, there is a lack of randomized controlled trials to draw the conclusion that all CAD patients should be investigated for OSA and subsequently be treated with continuous positive airway pressure (CPAP). Objective. The Randomized Intervention with CPAP in CAD and OSA (RICCADSA) trial is designed to address if CPAP treatment reduces the combined rate of new revascularization, myocardial infarction, stroke and cardiovascular mortality over a 3-year period in CAD patients with OSA. Secondary outcomes include cardiovascular biomarkers, cardiac function and maximal exercise capacity at 3-month- and 1-year follow-ups. Patients and methods. A sample of 400 CAD patients (100 non-sleepy OSA patients randomized to CPAP, 100 to non-CPAP; 100 sleepy OSA patients on CPAP, and 100 CAD patients without OSA) will be included. So far, 240 patients have been enrolled in the trial since December 31, 2005. Conclusion. The RICCADSA trial will contribute to defining the impact of CPAP on prognosis of CAD patients with OSA.
Rationale
Obstructive Sleep Apnoea (OSA) in Coronary Artery Disease (CAD)
Obstructive Sleep Apnoea is characterized by intermittent episodes of upper airway collapse during sleep, associated with increasing respiratory efforts and increased cardiac oxygen demand on one hand and low oxygen reserve due to lack of ventilation on the other. OSA has been observed in almost one third of patients with CAD Citation1. The prognosis for CAD patients with concomitant OSA was found to be worse than the prognosis for patients without OSA Citation2, Citation3. A recent 6 month follow-up study found OSA in more than half of the CAD patients undergoing Percutaneous Coronary Intervention (PCI), and the incidence of major adverse cardiac events (cardiac death, reinfarction and target vessel revascularization) was almost 24% among the patients with concomitant OSA compared with 5% among those without OSA Citation4. Moreover, a recent retrospective review of 371 OSA patients undergoing PCI suggested a significantly higher cardiac death rate among untreated OSA patients than the treated subjects at 5 years Citation5. However, there is also controversy demonstrating no significant difference regarding 10-year survival in CAD patients with OSA compared with those without OSA at baseline Citation6.
CAD in OSA
Regarding CAD prevalence in OSA, a significant relationship has also been suggested in the literature Citation7. For instance, in a sleep clinic cohort of 386 subjects Citation8 CAD was present in almost one quarter of OSA subjects and the percentage of patients with CAD was high among those with moderate to severe OSA. However, clinical studies of CAD in sleep clinic cohorts generally involve OSA patients with daytime symptoms. Consequently, in contrast to the data from the general population these studies selectively deal with symptomatic patients. This group is more likely to suffer from more severe sleep apnoea, potentially to be more obese and to present with other cardiovascular comorbidity. Available cross-sectional data from sleep clinic cohorts is, to date, therefore neither representative for all OSA subjects nor reliable enough to suggest a causal relationship between OSA and CAD.
Is OSA a risk factor for CAD?
Longitudinal studies are in that concept of more scientific importance. For instance, an observational follow-up study of a middle-aged sleep clinic cohort, clinically free of coronary symptoms at the time of OSA diagnosis, revealed an almost five-fold relative risk of incident CAD during a seven-year period Citation9. Moreover, more than 50% of normotensive men not treated for OSA developed at least one cardiovascular disease during the follow-up period with new cases of CAD also among those maintaining normotension Citation10, suggesting that CAD development in part may be independent of systemic hypertension. A larger observational study of a sleep laboratory cohort with a mean follow-up of 10 years found a three to four times higher incidence of fatal and non-fatal cardiovascular events in patients with severe OSA compared with simple snorers Citation11. However, despite a significant association between OSA and CAD in clinical cohorts after adjustment for confounding factors and the evidence from the few longitudinal incident CAD studies, this relationship is weaker in the cross-sectional analysis of the Sleep Heart Health Study cohort data Citation12.
A need for a randomized controlled trial (RCT)
Elimination of obstructive apnoeas and hypopnoeas with nasal continuous positive airway pressure (CPAP) is the first line treatment, resulting in reduced daytime sleepiness and improved quality of life for OSA patients Citation13. However, the majority of patients with CAD with concomitant OSA do not experience daytime sleepiness, i.e. they are asymptomatic, and therefore, are not recognized and considered for OSA treatment with CPAP. There is a lack of RCTs which makes it impossible to draw the conclusion that all CAD patients should be investigated for OSA and subsequently be treated.
The rationale for considering CPAP for CAD patients with concomitant OSA regardless of daytime sleepiness is based on the adverse hemodynamic changes observed during the obstructive events Citation7, which might be reversed by the physiological effects of the device. In general, the apnoea event leads to increased work of breathing, recurrent episodes with considerable negative intrathoracic pressure, hypoxia/reoxygenation, and fluctuating autonomic activity with considerable oscillations in heart rate and blood pressure. Increased oxygen demand and reduced oxygen supply during episodes with obstructive events may trigger an attack of angina pectoris in patients with CAD who already had a reduced coronary flow reserve Citation14. OSA is also associated with long-term alteration of cardiac structure, hemodynamic reflex function and vascular structure/function. The disorder leads to immediate and sustained sympathetic activation Citation15. Baro- and chemoreceptor responsiveness is altered Citation16 and vascular reactivity in terms of responsiveness to hypoxemia or vasoconstrictors appears to be elevated Citation17. A series of recent studies have demonstrated that vascular endothelial function is reduced in OSA Citation18. All these changes are specific to OSA in the sense that they might be reversed by CPAP Citation19.
Due to the serious acute cardiovascular responses during obstructive events, it has been argued not to design long-term randomized controlled trials in OSA patients for ethical considerations Citation1. However, as long-term follow-up studies do not always support an adverse impact of the disorder and are not prospectively controlled for the confounding factors such as high age, obesity, insulin resistance, hyperlipidemia, smoking and life-style habits, a real causal relationship between OSA and CAD has yet not been readily confirmed. Moreover, long-term adherence to CPAP treatment in CAD patients with concomitant OSA without daytime sleepiness has yet not been proven either. Indeed, one recent study Citation20 addressed the issue in a sleep clinic cohort in OSA subjects with CAD, suggesting a comparable compliance between sleepy and non-sleepy patients at a one-year follow-up. As the patients were recruited from a sleep clinic cohort, these subjects had, anyhow, some form of complaints, though not significant daytime sleepiness, and could therefore have benefited from the CPAP treatment. The situation in a cardiac clinic population is quite different, as the subjects themselves are not actively seeking for a referral but being considered for a screening procedure. Thus, OSA is diagnosed as a “laboratory” rather than a “clinical” disorder. For those CAD patients who do not have complaints related to OSA symptoms, it may therefore constitute a challenge to convince them to be treated “by a mask on the face” as a kind of “chronic cardiovascular treatment”. The issue becomes even more complicated if such an intervention would adversely affect the patient's quality of life. Thus, to date, to the authors’ best knowledge, despite a very high occurrence of OSA in CAD patients, these high risk subjects are not routinely being screened for OSA by cardiologists, and treatment with CPAP is not being considered either in the clinical practice.
The current randomized intervention with CPAP in CAD and OSA (RICCADSA) trial has therefore been designed to contribute to resolving some of these controversies.
Objectives
The primary aim of the RICCADSA trial is to address whether CPAP treatment of newly revascularized CAD patients with OSA without daytime sleepiness will reduce the combined rate of cardiovascular mortality, stroke, myocardial infarction and the need for a new revascularization in these subjects over a three-year period. Secondary endpoints include cardiovascular biomarkers, left ventricular function, maximal exercise capacity, quality of life, anxiety and depression state at 3-month- and 1-year follow-ups in relation to baseline findings as well as sleep architecture and OSA severity on polysomnography (PSG) at baseline and adherence to CPAP therapy in OSA patients during the follow-up period. Moreover, acute hospital admissions over a 3-yr period will be documented for the whole group.
The RICCADSA trial protocol
The RICCADSA trial is a prospective, randomized, controlled, open-labeled, blinded evaluation type of a clinical trial, which started in 1 December 2005. While the patients cannot be blinded to this intervention because no true neutral sham CPAP placebo is available, all outcome measurements will be performed and interpreted by personnel unaware of the allocated treatment. The trial duration is estimated to be six years for the primary outcome measures. The goal is to enrol 400 patients over the first three years, and to follow them up for a minimum of three years.
The study population
The patient inclusion and exclusion criteria are shown in , and the study protocol is outlined in . All consecutive patients are recruited from two hospitals (Skövde and Lidköping) with training and research facilities serving a population of around 250 000 living in the Skaraborg County of West Götaland. Acute care for cardiovascular patients is carried out in both hospitals with access to the intensive care units. PCI is performed either as a planned or rescue intervention at the hospital in Skövde during daytime. Rescue PCI as well as Coronary Artery Bypass Grafting (CABG) surgery is otherwise performed at the Sahlgrenska University Hospital in Gothenburg, which is the regional hospital. All patients are warded at the study hospitals, respectively, after the revascularization when the clinical state is considered stable. The Coronary Artery Out-Patient Clinics (CAOPC) at the hospitals in Skövde and Lidköping provide access to a database for all subjects undergoing coronary angiography as well as PCI or CABG in the Skaraborg county. All eligible patients are being informed by the special nurses at the CAOCs about the RICCADSA trial and asked if they wish to participate in the study after they have been given both oral and written information. The patients are then referred to the Sleep Apnoea Out-Patient Clinic (SAOPC) in Lidköping or the Sleep Medicine Unit (SMU) in Skövde for an unattended sleep recording at home by a portable cardiorespiratory polygraphy device (Embletta®, Flaga, Reykjavik, Iceland) within the six months following the revascularization. Patients with an estimated sleep time of less than four hours are offered a new home-based sleep study. All baseline-screening recordings are scored by the same observer for decision making according to the inclusion and exclusion criteria (see below). Apnoeas are defined as complete cessation of airflow and hypopnoeas with a reduction in thoracoabdominal movement ≥50% and/or in the nasal pressure amplitude of ≥50% for ≥10 seconds. The patients with an apnoea-hypopnoea index (AHI) ≥15 per hour of estimated sleep time independent of occurrence of symptoms are defined as OSA. The subjects with dominantly (more than 50%) central apnoeas and hypopnoeas of Cheyne-Stokes nature are not included in the study. The CAD patients with AHI≤ 5 per hour of estimated sleep time are defined as non-OSA and included in the trial as an additional control group (). The subjects with AHI of at least 5 and less than 15 per hour are defined as borderline OSA and handled according to the clinical routines, i.e., are informed about the results and offered CPAP treatment if they have daytime sleepiness. The borderline group is not included in the trial.
Figure 1. Outline of the randomized intervention with CPAP in coronary artery disease and obstructive sleep apnoea (RICCADSA) trial protocol. CPAP: Continuous Positive Airway Pressure; CAD: Coronary Artery Disease; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Grafting; ESS: Epworth Sleepiness Scale; OSA: Obstructive Sleep Apnoea; AHI: Apnoea-Hypopnoea-Index.
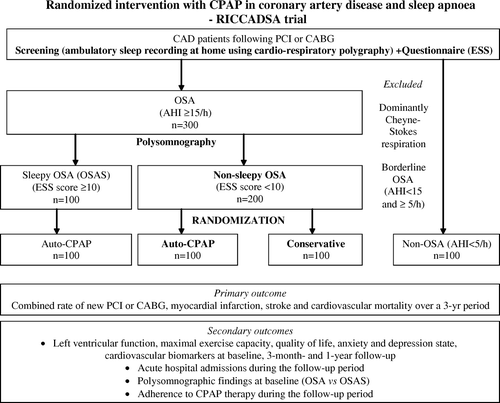
Table I. Eligibility criteria for the randomized intervention with CPAP in Coronary Artery Disease and Obstructive Sleep Apnoea – RICCADSA trial
Baseline assessments
All CAD patients with OSA diagnosis on the first screening investigation by Embletta system undergo an unattended overnight PSG using a computerized recording system (Embla A10©, Flaga, Reykjavik, Iceland) at the SMU in Skövde for a more proper sleep evaluation and further comparisons between the sleepy and non-sleepy OSA patients. PSG recordings are scored by an observer blinded to the clinical data and baseline screening results from the previous Embletta recordings. Following the PSG night at the SMU, after an at least 10-hours-fast, venous blood samples are drawn between 07.00-08.00 in the morning for measurement of hemoglobin, hematocrit, white blood cells, trombocytes, glucose, total cholesterol, low density as well as high density lipoprotein cholesterol, triglyceride, C-reactive protein and N-terminal pro-brain natriuretic peptide levels. Additional blood samples are centrifuged and stored in small portions at -80°C for future analysis of cardiovascular biomarkers.
At baseline, clinical examination is performed and the patients are given oral information about the trial once again. The coronary angiography data before the revascularization as well as smoking habits, other comorbidity data and medications are documented in all trial subjects. Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Short- Form (SF)-36 Quality of Life, Zoung Anxiety as well as Depression Scale questionnaires are obtained, and a transthoracal echocardiography (TTE) is performed before the start of the intervention procedure.
CAD patients without OSA also follow the same procedure except an overnight polysomnography as AHI less than 5 per hour upon the Embletta system has a reliable sensitivity and specificity to exclude OSA Citation21. Moreover, a detailed sleep structure of the non-OSA subjects is not considered to have a major importance for the rationale of the current trial.
Random assignment
Allocation of the OSA patients to randomization to CPAP or control group or start of CPAP treatment without randomization relies on the subjective ESS score Citation22. CAD patients with OSA and an ESS score of at least 10 are regarded as symptomatic (sleepy) sleep apnoeics and are offered CPAP treatment (). The patients with OSA having an ESS score below 10 are defined as asymptomatic (non-sleepy) sleep apnoeics and are therefore randomized to CPAP or control group.
Random assignment is scheduled by random numbers with a block size of eight patients (four CPAP and four controls in each block) stratified according to gender and the type of revascularization in order to obtain an equal distribution of men and women as well as PCI and CABG intervention in the groups. The first patient was recruited in December 2005.
Initiation of CPAP
Symptomatic OSA patients who are offered treatment as well as asymptomatic OSA patients who are randomized to CPAP intervention are informed about the technical procedure in the morning following the PSG evaluation and equipped with an automatic (self-titrating) CPAP device (S8®, ResMed, Sydney, Australia) with a nasal or full-face mask and humidifier by trained staff at the SMU. All patients assigned to CPAP are instructed to use CPAP at home every night for at least 4 hours, contacted by telephone after one week and given a check-up in the clinic after 1 month, 3 months, 6 months and 1 year, respectively.
Asymptomatic OSA patients who are randomized to the control group are advised weight reduction if they are overweight and informed about the tennis ball technique to avoid supine position during sleep Citation23.
Follow-up
The follow-up procedure includes clinical assessments at three months, one year, two and three years, respectively, after the baseline investigation for each patient. A new sleep recording with the Embletta system is performed at three months, 1 year, 2 years and 3 years, respectively in all patients with OSA (with concomitant CPAP treatment for the ones assigned to the device) at the SMU. Questionnaires are assessed, blood samples are taken in the morning following the new sleep recording, and a new TTE is performed as described above. In addition, a maximal exercise testing is performed at three months and one year following the baseline investigations.
For the non-OSA group, no blood samples are drawn at three months but questionnaires, TTE and maximal exercise testing are performed accordingly at three months and one-year follow-ups. Blood samples are drawn in the non-OSA group at one-year follow-up. New sleep recording with the Embletta system is performed at 1 year, 2 years and 3 years also in this group in order to ensure that they are still free of OSA.
All CAD patients are given necessary cardiovascular drug treatment by their physicians regardless of the current CPAP trial and medical changes are reported in the charts in the clinical follow-up period.
Compliance
The patients bring their CPAP devices to the clinic at each scheduled follow-up visit, and the monitoring settings as well as the hours of CPAP use are recorded. Moreover, CPAP pressure, mask leak and residual AHI measures are registered. The information from the sleep recording with the Embletta system at the follow-up night is taken into consideration in evaluation of the treatment efficiency of the CPAP device. All necessary adjustments of the CPAP device and mask fittings are done according to the clinical routines by the SMU staff. The patients who are unable to adhere with the CPAP device are followed-up accordingly in the treatment arm in conformity with the predetermined intention-to-treat analysis.
Primary outcome assessments
The primary outcome is the combined rate of cardiovascular mortality, stroke, myocardial infarction and the need for a new revascularization over a three-year period in the study population. Information will be obtained from patients’ medical records, and when necessary, from the Swedish Hospital Discharge Register as well as National Cause of Death Registry.
Secondary outcome assessments
Secondary outcomes include cardiovascular biomarkers, left ventricular function, maximal exercise capacity, quality of life, anxiety and depression state at baseline, 3-month- and 1-year follow-ups as well as PSG findings at baseline, adherence to CPAP therapy and acute hospital admissions during the follow-up period.
Sample size estimation
The combination of cardiovascular mortality, acute myocardial infarction and the need for a new revascularization was reportedly 27% within a year of the PCI Citation24. In a recent systemic review of comparative effectiveness of PCI and CABG, the repeated revascularization was suggested to be 40.1% in PCI with stents and 9.8% in CABG patients at 5 years Citation25. To the our best knowledge, there is lack of prospective data regarding long-term outcome for revascularized CAD patients with OSA, and it is therefore difficult to carry out a real power calculation. Hypothetically the prognosis should be expected to be worse for this subgroup with regard to earlier studies of the patients with CAD and concomitant OSA Citation2 due to pathophysiological cardiovascular load with increased sympathetic activity and recurring episodes of oxygen deficit in these individuals. As no previous studies allow us to make a proper power estimate for the primary outcome assessments, we hypothesized a composite end-point rate of 25% in the asymptomatic sleep apnoeics without CPAP treatment over a three-year follow-up period. The trial will comprise a consecutive sample of 400 CAD patients (100 non-sleepy OSA patients randomized to CPAP, 100 to non-CPAP; 100 sleepy OSA patients [ESS > = 10] on CPAP, and 100 CAD patients without OSA [AHI <5 per h]). Approximately 25% of the non-sleepy OSA subjects may be non-compliant with the therapy during the follow-up period. For the main study group, i.e., the non-sleepy sleep apnoeics, the trial is expected to have an 80% power to detect a risk reduction from an assumed composite end-point rate of 25 to 10% for the primary outcome at intention-to-treat basis (p < 0.05 level, two-sided test). The number of sleepy sleep apnoeics on CPAP treatment as well as of CAD patients without OSA (100 for each) is chosen for comparable statistical calculations between the subgroups.
Data collection and analysis
The primary outcome variables are prospectively being documented and are not subject to observer bias. Baseline comorbidity data, results of the sleep recordings, compliance with CPAP device, scores of the questionnaires, results of the blood analysis as well as TEE and maximal exercise testing are performed and/or documented consecutively in separate files at a specific server of the study hospital by research personnel blinded to the allocation of the study groups and/or unaware of the results of the concomitant outcomes.
Demographic and clinical data will be expressed as mean ± SD for continuous variables. The primary statistical analysis will be based on the intention-to-treat principle. A stratified log-rank statistic will be used to compare the event free survival curves for all four groups. The Kaplan-Meier method will be used to construct life-table plots. Secondary analyses will also be conducted, including “on treatment” analysis of only those randomly assigned to CPAP, who have continued to use it. For this, data from patients who have crossed over will be censored at the time of cross over from the final analysis. Multivariate analyses using Poisson regression models will address issues about interactions such as those between treatment and the variables time since inclusion, concomitant cardiovascular drug treatment, CPAP compliance and baseline variables on the outcome measures. As significant between-group differences in mortality alone may occur, the survival time until death or the end of the trial will also be analyzed. Data will also be analyzed separately according to PCI or CABG intervention subgroups.
Secondary outcomes reflected by continuous variables will be compared between the groups by ANOVA using baseline measurements as covariates. Other secondary outcomes will be compared by using logistic regression analysis. The data of patients without follow-up measurements will be censored from the analyses of secondary outcomes. Non-parametric methods will be used to compare the number of acute hospital admissions and the number of days spent in hospital due to cardiovascular reasons in the groups. Baseline data from dropouts will be analyzed separately to see if these patients have differed in any important way from patients who have remained in the trial for its entire duration.
Substudies
Polysomnographical characteristics of the sleepy vs. non-sleepy sleep apnoeics as well as possible differences in adherence to CPAP treatment between these groups will also be addressed in substudies. Moreover, in-home monitoring of CAD patients with Embletta system will be compared with the PSG results, which will provide information about the effectiveness of the Embletta system as a screening tool for OSA diagnosis in cardiac patients.
Ethical considerations
The study protocol was approved by the Ethics Committee of the Medical Faculty of the University of Gothenburg (nr 207-05; 09.13.2005), and a written informed consent is obtained from the patients before entering the study.
Register
The study was registered at the national research website in Sweden through the database of the Skaraborg Hospital (nr VGSKAS-4731; 04.29.2005) as well as with ClinicalTrials.gov, number NCT00519597.
Conclusion
Our primary end point in this on-going RCT is the combined rate of cardiovascular mortality, stroke, myocardial infarction and the need for a new revascularization. If CPAP reduces this rate significantly in the non-sleepy sleep apnoeics, our results would have immediate clinical application to actively screen CAD patients for OSA and to initiate treatment with CPAP in those having OSA regardless of daytime sleepiness. Consequently, these results may improve treatment and risk stratification for CAD patients, and thus, may also have important health economic implications. If, on the other hand, results from the RICCADSA trial do not support CPAP treatment of OSA patients without daytime sleepiness, such individuals in cardiac clinics would not need to be considered for an unnecessary investigation and ineffective treatment.
By May 31, 2008, so far, 350 CAD patients undergoing coronary revascularization have been screened and 240 enrolled in the RICCADSA trial.
Acknowledgements
The trial is supported by the Swedish Heart-Lung-Foundation, Research Fund at Skaraborg Hospital, Heart Foundation of Karnsjukhuset, and ResMed Ltd.
The paper was presented at the annual meeting of the European Respiratory Society in Stockholm on 18th September 2007.
References
- Peker Y, Kraiczi K, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J 1999; 14: 179–84
- Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory Disturbance Index: An independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med 2000; 162: 81–6
- Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: Long-term prognosis. Am J Respir Crit Care Med 2001; 164: 1910–3
- Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnoea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007; 99: 26–30
- Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnoea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol 2007; 50: 1310–4
- Hagenah GC, Gueven E, Andreas S. Influence of obstructive sleep apnoea in coronary artery disease: A 10-year follow-up. Respir Med 2006; 100: 180–2
- Hedner J, Franklin K, Peker Y. Obstructive sleep apnoea and coronary artery disease. In: Kryger, Roth, Dement. Principals and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Inc; 2005. p 1203–7.
- Maekawa M, Shiomi T, Usui K, Sasanabe R, Kobayashi T. Prevalence of ischemic heart disease among patients with sleep apnoea syndrome. Psychiatry Clin Neurosci 1998; 52: 219–20
- Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur Respir J 2006; 28: 596–602
- Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnoea: A seven-year follow-up. Am J Respir Crit Care Med 2002; 166: 159–65
- Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcome in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005; 365: 1046–53
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001; 163: 19–25
- Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax 1998; 53: 341–5
- Franklin KA, Nilsson JB, Sahlin C, Naslund U. Sleep apnoea and nocturnal angina. Lancet 1995; 345: 1085–7
- Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension?. J Hypertension 1988; 6(Suppl 4)529–31
- Ryan S, Ward S, Heneghan C, McNicholas WT. Predictors of decreased spontaneous baroreflex sensitivity in obstructive sleep apnoea syndrome. Chest 2007; 131: 1100–7
- Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnoea. J Appl Physiol 2000; 89: 493–8
- Carlson JT, Rangemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertension 1996; 14: 577–84
- Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 2003; 177: 385–90
- Sampol G, Rodés G, Romero O, Jurado MJ, Lloberes P. Adherence to nCPAP in patients with coronary disease and sleep apnoea without sleepiness. Respir Med 2007; 101: 461–6
- Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J 2003; 21: 253–9
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991; 14: 540–5
- Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnoea patients: A 6-month follow-up study. Laryngoscope 2006; 116: 1995–2000
- Odell A, Gudnason T, Andersson T, Jidbratt H, Grip L. One-year outcome after percutaneous coronary intervention for stable and unstable angina pectoris with or without application of general usage of stents in unselected European patient groups. Am J Cardiol 2002; 90: 112–8
- Bravata DM, Gienger AL, McDonald KM, Sundaram V, Perez MV, Varghese R, et al. Systematic review: The comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med 2007; 147(20)703–16