Abstract
Objective. To determine whether T-wave axis on the resting electrocardiogram (ECG) is associated with coronary artery calcification (CAC) score, a measurement of coronary atherosclerosis, in older adults. Methods. The sample consisted of 2004 adults aged 66 and over participating in the prospective, population-based Age-Gene/Environment Susceptibility-Reykjavik Study. The cohort was divided into gender-stratified quartiles based upon Agatston CAC score derived from computerized tomography. Frontal T-axis deviation from 45° was assessed using surface ECG. Statistical analysis was performed with Tobit regression models adjusted for demographic and cardiovascular risk factors. Results. In the entire study population, T-axis deviation from 45° was significantly associated with increasing CAC score in men (p<0.001) and women (p=0.03). In men without clinically recognized coronary heart disease (CHD), the association with CAC score remained statistically significant (p=0.02). This association was significant among women without CHD once male CAC cut points were used (p=0.05). Conclusion. An abnormal T-wave axis is associated with an increasing CAC score in this population of Icelandic older adults. This association remains in the subgroup of subjects without clinical CHD.
The axis of the T-wave on the electrocardiogram (ECG) is a marker of ventricular repolarization that, when deviated from normal by more than 60 degrees in either direction in the frontal plane, has been strongly associated with an increased risk of fatal and non-fatal cardiac events in older adults Citation[1] as well as all-cause mortality, incident coronary heart disease (CHD), and death from CHD in older adults free of CHD at baseline Citation[2]. Abnormalities in ventricular repolarization on ECG have long been recognized as features of myocardial ischemia. However, the pathophysiologic relationship between CHD and deviation of the T-wave axis from normal is unclear, especially in light of data showing a lack of association between an abnormal T-axis and coronary atherosclerotic disease on angiography Citation[3].
Coronary artery calcium (CAC) score is a measure of coronary atherosclerosis assessed by computerized tomography (CT). CAC score is strongly correlated with angiographic and histopathologic evidence of coronary atherosclerosis Citation4–7 and is an independent predictor of coronary events, even in adults over the age of 70 Citation[8]. While CAC scoring is not designed to localize which regions of the myocardium are at greatest risk of ischemia or infarct, nor is it a direct measurement of coronary stenosis, it is useful in providing a global assessment of CHD risk. To assess the degree to which T-axis is associated with underlying coronary atherosclerosis, we analyzed the association between CAC score and degree of T-axis deviation from normal. We carried out this analysis in the Age Gene/Environment Susceptibility (AGES)-Reykjavik Study population, first in the entire cohort, then in a subpopulation free of clinical CHD.
Methods
Study population
AGES-Reykjavik is a prospective, population-based study of older adults born between 1907 and 1934 and residing in Reykjavik, Iceland. The study is designed to assess neurocognition, musculoskeletal function, body composition, and cardiovascular function in older adults. It is an extension of the Reykjavik Study, a prospective study of a random sample of Icelandic men and women intended to investigate the natural history of heart disease. Study methods have been described previously Citation[9]. AGES-Reykjavik was approved by the National Bioethics Committee and the institutional review boards of the Icelandic Heart Association and National Institute on Aging. All subjects gave informed consent.
This analysis was performed on the participants (n = 2300; 976 men, 1 324 women) recruited for the baseline study of AGES-Reykjavik between 2002 and 2004. Data collected included medical history and current medications, answers to the Rose angina questionnaire Citation[10], blood sample, resting ECG, and multi-detector CT for CAC scoring. Subjects with incomplete ECG or CT data (n = 296) were excluded, leaving a study population of 2004 (848 men, 1 156 women).
In the second analysis, we tested our hypothesis in a population free of CHD by excluding subjects with a positive score for angina by the Rose criteria (n = 50), self-reported history of percutaneous coronary intervention (n = 165), coronary artery bypass graft (n = 157), myocardial infarction (MI) (n = 363), or silent Q waves on ECG (Minnesota codes 1.1–1.2, n = 43), or an unknown MI/coronary intervention history (n = 56). Subjects with right or left bundle-branch block, left anterior hemiblock, or intraventricular block (Minnesota codes 7.1, 7.2, 7.4, or 7.7 n = 166) also were excluded from the second analysis because ventricular conduction abnormalities are known to affect the T-wave and may be secondary to underlying ischemia. The final population free of CHD or ventricular conduction defects was 1433 (511 men, 922 women).
ECG outcome
Resting 12-lead ECGs were recorded using a Marquette/MAC 5000 ECG machine (General Electric Marquette Medical Division, Milwaukee, Wisconsin, USA). Patients were at rest and supine and the electrodes were placed in the standard positions. A technician recorded a 10 second ECG digitized at a rate of 500 samples per second. It was analyzed using the 12 SL analysis program (General Electric Marquette Medical Division, Milwaukee, Wisconsin, USA) and manually by expert readers and coded according to the Minnesota codes Citation[11].
Frontal T-axis was calculated by the 12SL program Citation[12]. Briefly, the program calculated the signed areas of the T-wave projection in leads I and II by calculating the algebraic sum of samples between the onset and the offset of the T-wave in these leads. The axis was calculated as follows: α = tan−1(2/√3((II-I/2)/I)) where α represents the T-wave axis in the frontal (XY) plane and I and II represent the signed areas of the vectors in leads I and II, respectively. T-wave axis was reported as the angle between the x axis and the axis of the T-wave in the frontal (XY) plane. The main outcome variable used in this analysis was the absolute value of frontal T-axis deviation from 45°. This value was chosen to facilitate comparisons with prior studies in which a frontal T-axis between 15° and 75° was defined as normal Citation[1]. QT interval was corrected for rate using Bazett's equation (QTC=QT interval(msec) /√(60/ventricular rate)). QRS-T angle was derived by subtracting the frontal QRS axis from the frontal T-axis, and the results were divided into gender-specific quartiles based upon an absolute value of the deviation from the population median of 31°. ST-segment depression was defined as Minnesota codes 4.1 or 4.2. Minor ST-segment change was defined as Minnesota codes 4.3 or 4.4. T-wave inversion was defined as Minnesota codes 5.1 or 5.2, and minor T-wave change was defined as Minnesota codes 5.3 or 5.4.
CT imaging of coronary calcium
Approximately two weeks after the clinic visit in which the ECG was taken, all participants were scanned in one imaging center using a Siemens Somatom Sensation 4 multi-detector CT (Siemens Medical Solutions, Malvern, Pennsylvania, USA) with prospective ECG triggering. The heart was scanned sequentially using 2.5 mm thick slices in the cranio-caudal direction with the subject in suspended inspiration. Scans were analyzed with calcium scoring software by one of five readers using the image analysis software used in the MESA study Citation[13]. Inter-observer variability assessment showed high average correlation between the five readers and the UCLA Center (r = 0.94) based on the re-analysis of 200 scans.
CAC was quantified using the Agatston method Citation[14]. Briefly, in order to calculate an Agatston score, intracoronary calcified lesions were identified on each slice and a score was computed for each lesion that multiplied the area of the plaque by a factor accounting for tomographic density. These values were summed across all of the slices to create a global score. Gender-specific quartiles of CAC from the entire cohort were used in all analyses.
Covariates
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as systolic blood pressure above 140 mmHg, diastolic blood pressure above 90 mmHg, or use of an antihypertensive medication for the treatment of hypertension. Diabetes was defined as self-report of history of diabetes, fasting plasma glucose concentration greater than 126 mg/dl, or use of an oral hypoglycemic medication or insulin. Hypercholesterolemia was defined as fasting total cholesterol greater than 240 mg/dl, fasting low-density lipoprotein greater than 160 mg/dl, or use of an antilipemic medication. Smoking status was determined by self-report and categorized as “current smoker,” “former smoker,” or “never smoked.”
Statistical analysis
Demographic characteristics, cardiovascular risk factors, and selected ECG criteria were compared across the gender-specific quartiles of CAC using a one-way ANOVA or Kruskal-Wallis test for continuous variables where appropriate and chi-squared test for categorical variables. Tobit regression analysis was performed using the absolute value of the deviation of frontal T-axis from 45° as the outcome variable with the four gender-specific quartiles of CAC as the main independent variable. The Tobit model assumes an outcome that is normally distributed but censored at zero. This is equivalent to the behavior of the absolute values of a normal distribution, which was the nature of the distribution of T-axis deviation from 45° in this population.
The models were first adjusted only for age, then for age, BMI, smoking status, presence of hypertension, diabetes, and hypercholesterolemia. To address the issue of different CAC distributions between men and women, our analyses were repeated in women using the cut points for CAC quartiles derived from the population of men. Logistic regression analysis was used to test the association between other ECG repolarization abnormalities and CAC. All statistical analyses were performed using Stata, version 9.2 (StataCorp, College Station, Texas, USA).
Results
Distribution of CAC scores
Overall, subjects with a history of CHD had higher CAC scores than those without CHD. Men with CHD had a median CAC score of 1 035 (mean 1 478), while men without CHD had a median CAC score of 339 (mean 717). Women with CHD had a median CAC score of 420 (mean 720); those without CHD had a median CAC score of 98 (mean 355). displays the distribution of CAC scores by gender-specific CAC quartile for the entire study population. As expected given the higher mean CAC scores in subjects with CHD and in men, exclusion of those with CHD resulted in greater exclusion of men than women from the study and a greater attrition of subjects from the higher CAC groups.
Table I. Median, range of CAC scores by gender-specific CAC quartile: AGES-Reykjavik study
Clinical and ECG characteristics by CAC quartiles
In men, older age, increased BMI, use of an antilipemic medication, current smoking, hypertension, CHD, and peripheral arterial disease were all associated with increased CAC score (). Lower diastolic blood pressure, lower total cholesterol, and lower LDL levels were all associated with higher CAC scores. In women, older age, higher systolic blood pressure, use of an antilipemic medication, diabetes, smoking status, hypertension, and CHD were all associated with higher CAC score. As in the men, there was a significant trend toward lower total cholesterol and LDL in women with higher CAC. Men with higher CAC had a significantly rightward-shifted frontal T-axis, increased frontal T-axis deviation from 45°, longer mean rate-corrected QT interval, wider mean QRS-T angle, and were more likely to have a ventricular conduction abnormality (). The women in the highest CAC quartiles had a significantly rightward-shifted frontal T-axis, increased frontal T-axis deviation from 45°, and were more likely to have an inverted T-wave or any ST-T change.
Table II. Demographic and risk factor data by gender-specific CAC quartile, entire population: AGES-Reykjavik study
Table III. Selected ECG criteria by gender-specific CAC quartile, entire population: AGES-Reykjavik study*
T-axis deviation by CAC quartiles
In the entire study population, increased CAC score was significantly associated with increasing frontal T-axis deviation from 45° in both men and women, whether adjusting for age only, or for age and other cardiovascular risk factors (). When the analysis was limited to subjects free of symptomatic CHD or ventricular conduction abnormality, the association between CAC score and frontal T-axis deviation from 45° remained statistically significant in men, but not in women (, Supplemental Figure). To test whether the lack of an association between CAC and T-axis deviation in women was due to the use of different gender-specific CAC cut points, we applied the male CAC cut points to the female population, which revealed a statistically significant association between CAC and T-axis, even after adjustment for the cardiovascular risk factors ().
Supplementary Figure. Mean frontal T-Axis deviation from 45° by CAC quartile, men free of CHD. Bars represent 95% confidence interval.
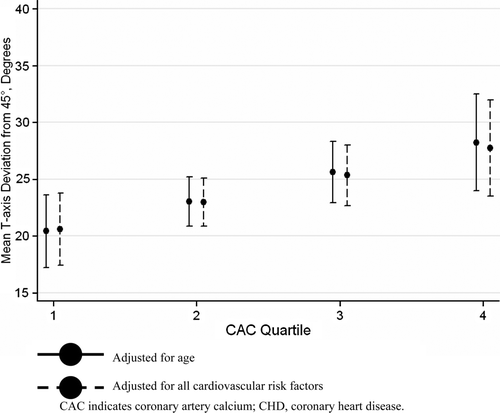
Table IV. Mean frontal T-axis deviation from 45° by CAC quartile
Other ECG repolarization abnormalities by CAC quartiles
Finally, we tested the relationship between CAC and several other indicators of abnormal repolarization on ECG (). In men, increasing CAC score was associated with QTc > 460 ms, deviation of the frontal QRS-T angle from normal, and minor T-wave changes. In women, inverted T-waves were associated with increasing CAC whether or not the male CAC cut points were used. After applying the male CAC cut points, having any ST-T change also showed an association with increasing CAC score that achieved statistical significance.
Table V. Odds (95%CI) for selected ECG criteria by increasing CAC quartile*
Discussion
In this population of older adults, increasing CAC score, a measurement of global coronary atherosclerotic burden, was associated with an increase in deviation of the frontal T-axis from 45°. In men, this association remained statistically significant after the exclusion of subjects with clinical CHD. In women free of CHD, the relationship between increasing CAC score and T-axis deviation was statistically significant once the male CAC cut points were applied. No other ECG indicator of repolarization abnormality that we tested was associated with high CAC in both men and women free of CHD. That the relationship between CAC and T-axis remained statistically significant in subjects without CHD suggests that T-axis deviation may be a marker of coronary atherosclerotic disease in individuals who are asymptomatic.
In two prior prospective studies, T-axis deviation from normal was associated with an increased risk of cardiovascular death, all-cause mortality, and incident CHD events Citation[1], Citation[2]. Although authors of those studies speculated that the abnormal T-axis was in part a reflection of myocardial ischemia, a separate study found no association between T-axis deviation and degree of angiographic evidence of atherosclerosis Citation[3]. However, this study used the Duke Myocardial Jeopardy Score, a measurement of myocardial area potentially at risk from upstream coronary artery stenosis, which is conceptually and physiologically different from Agatston scoring, a measure of coronary atherosclerotic burden. Therefore, the pathophysiologic link between coronary atherosclerosis and these changes in the T-wave axis remains to be elucidated.
In a separate population-based study assessing the relationship between CAC and ECG parameters, men without a CHD history with an abnormal T-axis had median CAC scores that were higher than those of men with any other repolarization abnormality Citation[15]. In two prior studies of middle-aged populations, T-axis deviation was not associated with fatal or non-fatal CHD events Citation[16], Citation[17]. However, in one of these studies, minor T-wave changes were associated with increased mortality from CHD and from all-cause cardiovascular disease Citation[16]. The authors of this analysis hypothesized that minor T-wave changes in midlife might precede T-axis deviation that could become apparent later in life.
We also found that frontal QRS-T angle was associated with increased CAC in men free of CHD. Other studies have found an association between spatial QRS-T angle and cardiovascular mortality Citation[18], Citation[19]. Although abnormalities of the QRS-T angle and T-axis are likely functions of the same underlying pathophysiology, the assessment of frontal T-axis offers several advantages from a public health perspective. While frontal QRS-T angle as we calculated it is only weakly correlated with spatial QRS-T angle Citation[20], frontal T-axis has been shown to have a similar predictive value for cardiac events whether assessed on surface ECG or on vectorcardiography Citation[1]. Frontal T-axis can be estimated easily by a clinician on routine ECG and also has a high degree of repeatability, two important attributes of any potential screening test Citation[21].
To address whether the evaluation of T-axis offers any information beyond the conventional assessment of changes in T-wave morphology, we repeated our analyses on a subset of our population without any abnormalities in the T-wave. In this analysis, the association between deviation in the T-axis from 45° and CAC remained statistically significant in men free of CHD, but not in women (data not shown). Others have noted that abnormalities in the spatial T-axis are not always reflected as inverted T-waves on the surface ECG Citation[2].
High CAC score has been shown to predict coronary events in older adults Citation[8] and has been associated with an increased risk of mortality in older men Citation[22]. However, coronary scanning by multi-detector CT is associated with significant cost and radiation exposure Citation[23], both of which detract from its utility as a screening tool in asymptomatic individuals. A recent joint consensus document from the American College of Cardiology Foundation and the American Heart Association made no specific recommendations regarding the use of CAC scoring by coronary CT in older adults Citation[24].
One additional finding worth noting was that in both men and women, participants in the highest CAC quartiles had lower total and LDL cholesterol levels, and men in the highest CAC quartiles had lower systolic and diastolic blood pressures. There are several potential explanations for this seemingly paradoxical association. First, a significantly higher proportion of subjects in the highest CAC quartile have CHD, so some of this paradox might be explained by behavioral and pharmacological changes (using an antilipemic or antihypertensive medication, for example) secondary to experiencing clinical CHD. Further, subjects with CHD typically have higher levels of circulating inflammatory cytokines, which may paradoxically lower cholesterol levels in these subjects Citation25–27. The lack of positive association between CAC and these well-studied risk factors for CHD in this population underscores the need for the development of new markers for CHD in older adults.
There are several potential limitations to our study. We chose to use an absolute value of T-axis deviation rather than consider leftward and rightward deviations separately because CAC scoring provides a global assessment of plaque burden. It is not designed to identify which areas of myocardium might be most at risk. Also, one potential reason for the difference in relationship between CAC and T-axis deviation between the entire cohort and the CHD-free subpopulation might have been the exclusion of subjects with ventricular conduction abnormalities on ECG from the latter group but not the former. However, when we adjusted our analyses in the entire cohort for the presence of these conduction defects, the results were similar to those reported. In addition, because left ventricular hypertrophy can also lead to repolarization abnormalities it is possible that this might confound the relationship between CAC and T-axis deviation. Although we adjusted our analysis for hypertension status, this may not have fully accounted for the effect of left ventricular hypertrophy on the T-wave axis.
In conclusion, we found that increasing deviation of the frontal T-axis from 45° is associated with increasing CAC score, a measure of coronary atherosclerosis, in this cohort of older adults, even after excluding subjects with CHD. Future work will be undertaken in the AGES-Reykjavik Study to determine the role of this ECG measurement in predicting CHD events in older adults free of clinical CHD. Especially given the somewhat paradoxical relationship between many of the major cardiovascular risk factors and CAC older adults, it will be useful to study what the assessment of the T-wave axis might contribute to coronary risk stratification in older adults.
Acknowledgements
This study was funded by NIH contract N01-AG-12100, the National Institute on Aging Intramural Research Program; Bethesda, MD, USA, Hjartavernd (Icelandic Heart Association); Kopavogur, Iceland, and the Althingi (Icelandic Parliament); Reykjavik, Iceland. Parts of this paper were presented as a poster presentation entitled “T-wave axis is associated with coronary artery calcium score in an asymptomatic elderly population” at the American Heart Association 47th Annual Conference on Cardiovascular Disease, Epidemiology, and Prevention, on March 1, 2007.
References
- Kors JA, de Bruyne MC, Hoes AW, van Herpen G, Hofman A, van Bemmel JH, et al. T-axis as an indicator of risk of cardiac events in elderly people. Lancet. 1998; 352: 601–5
- Rautaharju PM, Nelson JC, Kronmal RA, Zhang ZM, Robbins J, Gottdiener JS, et al. Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study). Am J Cardiol. 2001; 88: 118–23
- Alagiakrishnan K, Beitel JD, Graham MM, Southern D, Knudtson M, Ghali WA, et al. Relation of T-axis abnormalities to coronary artery disease and survival after cardiac catheterization. Am J Cardiol. 2005; 96: 639–42
- Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995; 92: 2157–62
- Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: A histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998; 31: 126–33
- Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: Results of 1,764 patients. J Am Coll Cardiol. 2001; 37: 451–7
- Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, et al. Coronary calcium measurements: Effect of CT scanner type and calcium measure on rescan reproducibility–MESA study. Radiology. 2005; 236: 477–84
- Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005; 112: 572–7
- Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Prevalence of coronary heart disease in Icelandic men –1986. The Reykjavik Study. Eur Heart J. ;14 1968; 1993: 584–91
- Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977; 31: 42–8
- Prineas R, Crow RS, Blackburn H. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Wright PSG, Boston 1982
- Farrell R. Axis calculation by the 12SL Program. General Electric Publication. 2005
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002; 156: 871–81
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15: 827–32
- Mohlenkamp S, Schmermund A, Lehmann N, Roggenbuck U, Dragano N, Stang A, et al. Subclinical coronary atherosclerosis and resting ECG abnormalities in an unselected general population. Atherosclerosis. 2008; 196: 786–94
- Prineas RJ, Grandits G, Rautaharju PM, Cohen JD, Zhang ZM, Crow RS. Long-term prognostic significance of isolated minor electrocardiographic T-wave abnormalities in middle-aged men free of clinical cardiovascular disease (The Multiple Risk Factor Intervention Trial [MRFIT]). Am J Cardiol. 2002; 90: 1391–5
- Vaidean GD, Rautaharju PM, Prineas RJ, Whitsel EA, Chambless LE, Folsom AR, et al. The association of spatial T-wave axis deviation with incident coronary events. The ARIC cohort. BMC Cardiovasc Disord. 2005; 5: 2
- Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003; 24: 1357–64
- Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005; 2: 73–8
- Rautaharju PM, Prineas RJ, Zhang ZM. A simple procedure for estimation of the spatial QRS/T angle from the standard 12-lead electrocardiogram. J Electrocardiol. 2007; 40: 300–04
- Vaidean GD, Schroeder EB, Whitsel EA, Prineas RJ, Chambless LE, Perhac JS, et al. Short-term repeatability of electrocardiographic spatial T-wave axis and QT interval. J Electrocardiol. 2005; 38: 139–47
- Abbott RD, Ueshima H, Masaki KH, Willcox BJ, Rodriguez BL, Ikeda A, et al. Coronary artery calcification and total mortality in elderly men. J Am Geriatr Soc. 2007; 55: 1948–54
- Hunold P, Vogt FM, Schmermund A, Debatin JF, Kerkhoff G, Budde T, et al. Radiation exposure during cardiac CT: Effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003; 226: 145–52
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation 2007; 115: 402–26
- Ettinger WH, Jr, Sun WH, Binkley N, Kouba E, Ershler W. Interleukin-6 causes hypocholesterolemia in middle-aged and old rhesus monkeys. J Gerontol A Biol Sci Med Sci. 1995; 50: M137–M140
- Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000; 343: 1139–47
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997; 336: 973–9