Abstract
Objectives. Annuloplasty is the most common surgical procedure for ischemic mitral regurgitation (MR) that improves symptoms but is also subjected to high incidence of recurrent MR. One of the reasons of recurrent MR could be further left ventricular (LV) remodeling. Design. The study population consisted of 195 patients with ischemic MR. Mitral valve repair and bypass surgery was performed between 2000 and 2006. Results. LV end diastolic diameter (LVEDD) increased in 30.3% of patients in one year following mitral repair. Multivariate ANOVA analysis revealed that if LVEDD index (LVEDDi) before surgery is less than 25 mm/m2, the probability for LVEDDi to diminish or to stay at the same range is 84.6% higher, than in the case of preoperative LVEDDi ≥25 mm/m2 and other predictive variables. Conclusions. Predictive factors for further LV remodeling after ischemic mitral repair 1 year after surgery are preoperative LVEDDi, preoperative LV end systolic diameter index, tricuspid regurgitation grade before surgery, and early postoperative MR grade.
Ischemic mitral regurgitation (MR) is a complication of chronic myocardial ischemia and infarction. Functional ischemic MR was defined as MR that occurs with a structurally normal valve due to LV systolic dysfunction and remodeling. The diagnostic criteria of ischemic MR are: MR occurring with one or more left ventricular (LV) segmental wall motion abnormalities, significant coronary disease in a territory supplying the wall motion abnormalities and structurally normal MV leaflets and chordae tendinae Citation1.
MR is a strong predictor of cardiac events independently of left ventricular systolic and diastolic indices in patients with ischemic heart disease. There is a graded positive association between the severity of MR and risk of death and heart failure. The presence of moderate MR (effective regurgitant orifice area 20 mm2) is associated with a > 3-fold risk of heart failure and > 2-fold risk of death at 5 years Citation2, Citation3. The presence of MR, even mild, carries an adverse prognosis due to the severe hemodynamic load on the post-infarcted ventricle. The presence of MR is a marker of the geometric abnormalities of the ventricle Citation1. Thus, the investigation of MR must be part of routine risk stratification and management planning in all post-MI patients.
Ischemic MR is also associated with poor long-term survival after CABG Citation4, Citation5. Thus, moderate- to severe and severe ischemic MR became obligatory surgical indication while performing CABG Citation6.Concerning the best management strategy for moderate ischemic MR the available data is conflicting, several reports supporting CABG alone Citation7–9 and several reports supporting CABG combined with mitral repair Citation10, Citation11. A significant reduction or elimination of MR after combined surgery has a marked positive impact on reverse LV remodeling, which is seen as a regression of ventricular dilatation, an increase in LVEF, and a decrease in PAP Citation12.
The restricted annuloplasty is the most common surgical procedure that improves heart failure symptoms but not the survival when compared to medical therapy and is also subjected to high incidence of recurrent mitral regurgitation. One of the reasons of recurrent mitral regurgitation could be continuing LV remodeling, with increasing left ventricular end diastolic diameter (LVEDD) after mitral repair. Otherwise, recurrent MR could be the reason of continuing LV remodeling. The present retrospective study was undertaken to evaluate the factors that could predict further LV remodeling after ischemic MR repair.
Material and methods
The study population consisted of 195 patients with ischemic heart disease and ischemic mitral regurgitation. Mitral valve (MV) repair and coronary bypass surgery (CABG) was performed at the Kaunas University Hospital between 2000 and 2006.
All patients underwent conventional multivessel CABG using mild hypothermic- normothermic cardiopulmonary bypass and antegrade cold crystalloid cardioplegia. All mitral valve repairs were performed by the same surgeon. The decision to treat the valve was at the discretion of the surgeon, the general indication being MR grade >2+. The mitral valve was approached via the right atrium and interatrial septum. The repair consisted of a suture annuloplasty. MV was approached via right atrium and interatrial septum. MV repair consisted of annuloplasty using double semi- purse string suture. Double semi-purse string suture technique consisted of placation-compression of MV annulus using Ethibond 2/0 parallel sutures, buttressed with perpendicular pledgets, each 0.8–1 cm in length, starting from fibrous trigons, along both commissures and meeting at the posterior mid-annulus. The achieved annulus diameter was measured with standard valve sizers. Double semi- purse string suture technique permitted to ensure asymmetrical remodeling of the annulus and achieve better accommodation of posterior leaflet P2, P3 scallops.
Baseline demographic and clinical characteristics of study population are presented in .
Table I. Baseline demographic and clinical data in study population.
Preoperative echocardiographic examinations were performed two days before surgery, early postoperative – 5–7 days after MV repair, and during a follow-up period of 1 year after operation.
Echocardiographic examination protocol included evaluation of left ventricular end diastolic diameter (LVEDD) and index (LVEDDi), left ventricular end systolic diameter (LVESD) and index (LVESDi), the diameter of left atrium (LA) in parasternal long axis view, evaluation of left ventricular ejection fraction (LVEF), left ventricular wall motion index (LV WMSi), right ventricular end diastolic diameter (RVEDD) and diameter of right atrium (RA) in apical four chamber view, sagital mitral annulus diameter, mitral regurgitation grade (MR), tricuspid regurgitation (TR) grade, pulmonary artery pressure (PAP) according to TR velocity (estimated by Bernoulli equation).
Taking into account the ischemic origin of MR and its different grading, the degree of MR was graded quantitatively using proximal isovelocity surface area (PISA) method for effective regurgitant orifice (ERO) calculations. ERO was the ratio of regurgitant flow to regurgitant velocity. MR was considered mild (1 + ), when ERO < 10 mm2, mild to moderate (2 + ) -ERO –10- < 20 mm2, moderate (3 + )- ERO 20–29 mm2 and severe ( 4 + )- ERO ≥ 30 mm2. MR grade was evaluated preoperatively in an awake person.
Statistical analysis was performed using SPSS 12.0 for windows. Multivariable ANOVA was used to determine the relationships between preoperative clinical or echocardiographic variables and postoperative LV remodeling. Results were expressed as mean±standard error mean. For comparison of variables before and after operation Student t test was used for parametric and Pearson χ2 for non parametric values. P<0.05 was considered as statistically significant.
Results
The study population was divided into two groups: group I-patients with increased LVEDD during one year after surgery (30.3% of study population) and group II-patients with the reduced or unchanged LVEDD (69.7% of study population) 1 year after surgery follow-up in comparison with preoperative value.
Mean age (65.45±0.96 years in group I and 65.15±0.83 years in group II (t = − 0.217, p = 0.829), gender (men constituted 79.2% of group I population and 74.6% of group II population (χ2=0.439, p = 0.508)), distribution of acute (18% in group I and 18.4% in group II (χ2=0.04, p = 0.949)) and previous myocardial infarction (relatively 90% and 86.9% (χ2=0.324, p = 0.569)) and preoperative mean congestive heart failure functional class (NYHA) (2.81±0.08 in group I and 2.86±0.04 in group II (t = 0.148, p = 0.882)) did not statistically significantly differ in two groups.
The mean number of diseased coronary arteries (2.57 ±0.12 in group I, 2.76±0.09 in group II (t = − 0.383, p = 0.702)) and mean number of grafts (3.13±0.07 in group I, 3.36±0.12 in group II (t = − 0.459, p = 0.647)) did not differ between the two groups.
Preoperative LVESD and LVESDi, MR severity, mitral annulus sagital diameter, LVEF, LV WMSi, LA and RA diameter, RVEDD, TR severity, PAP did not differ between the groups (p > 0.05) ().
Table II. Preoperative echocardiographic data.
LVEDD before surgery was significantly higher in group II than in group I ().
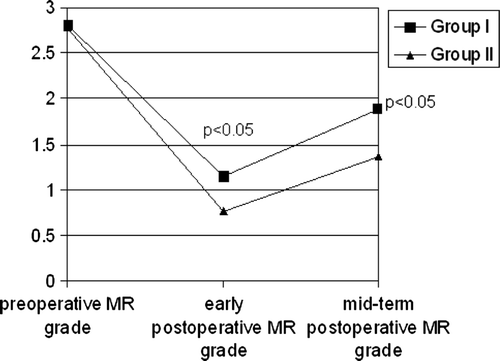
Early postoperative MR was significantly higher in group I in comparison with group II (respectively 1.15±0.11 and 0.76±0.07, t = − 2.98, p = 0.03) ().
Figure 1. Mean mitral regurgitation grade in different periods of examination.Differences of mean mitral regurgitation grade between two groups in early postoperative and 1 year after surgery are statistically significant (p < 0.05).Abbreviations: MR – mitral regurgitation.
One year after surgery LV ejection fraction increased (t = − 3.116, p = 0.002), LV wall motion score index (t = 2.651, p = 0.01), LA diameter (t = 5.763, p = 0.000) and congestive heart failure functional class (NYHA) decreased significantly (t = 7.473, p = 0.000) in group II. There were no significant changes in LV ejection fraction (t = − 1.728, p = 0.09) and LV wall motion score index (t = 0.014, p = 0.989), LA diameter (t = 0.336, p = 0.738), and congestive heart failure functional class (NYHA) (t = 0.668, p = 0.509) in group I.
One year after surgery mean LVEDD and LVEDDi, LVESD and LVESDi, LA, mitral annulus sagital diameter, MR grade, LV WMSi and mean congestive heart failure functional class (NYHA) (group I-2.85±0.01 and group II-2.3±0.07, t = − 4.253, p = 0.000) were significantly (p < 0.05) higher in group I ().
Table III. Postoperative echocardiographic data.
Multivariate ANOVA analysis revealed that LVEDD one year after surgery depends on these variables:
Preoperative LVEDDi (p < 0.001)-if LVEDDi before surgery is less than 25 mm/m2, the probability for LVEDDi to diminish or to stay at the same range is 84.6% higher, than in the case of preoperative LVEDDi ≥ 25 mm/m2.
Preoperative LVESDi-higher preoperative LVESDi at early postoperative period is associated with the higher LVEDDi 1 year after surgery (p = 0.003).
Preoperative TR grade- when TR grade before surgery is ≥ II°, the probability for LVEDDi to be more or equal 25 mm/m2 is 1.5 times greater than in the case of TR grade ≤ II° (p = 0.042)
Early postoperative MR grade. Higher MR grade at early postoperative period is associated with the higher LVEDDi 1 year after surgery (p = 0.033).
Discussion
Multiple pathophysiologic mechanisms, such as LV remodeling and systolic dysfunction, annular dilation, and mechanical asynchrony, are involved in generating ischemic MR, each of them having different weight Citation13. The presence of local or global LV remodeling can cause alteration in geometrical relationship between the ventricle and valve apparatus. Chronic ischemic MR is the consequence of a restriction in leaflet motion due to tethering by subvalvular apparatus Citation14. The mitral annular dilation and/or dysfunction, LV systolic dysfunction, and more recently the mechanical assynchrony of LV seem to have additional roles as modulating factors of the degree of MR. The tethering produces a leak in the MV both by causing a lack of coaptation due to the restricted leaflet motion and by creating a change in the geometry of the posterior leaflets with a consequent interscallop malcoaptation. LV asynchrony decreases the LV contraction efficiency and the closing forces, thereby generating an impairment of MV tenting Citation13.
According to our earlier published retrospective study, a significant reduction or elimination of MR after combined surgery has a marked positive impact on reverse LV remodeling, which is seen as a regression of ventricular dilatation, an increase in LVEF, and a decrease in PAP Citation12.
A standard surgical approach to treat ischemic MR is a ring or suture annuloplasty Citation15, which reduces mitral annular area by bringing the dilated posterior annulus anteriorly to reduce the anterior-posterior dimension and bring the leaflets into apposition. The aim of this procedure is to increase leaflet coaptation by correcting annular dilation. The ultimate goal of the annular procedure is to obtain LV reverse remodeling as a consequence of the disappearance of MR and LV volume overload Citation13.
Even though this procedure is initially effective in correcting ischemic MR and obtaining LV reverse remodeling in a large proportion of patients, a recurrent mitral regurgitation is found in about 30% of patients in late period after repair Citation16. Recurrence of ischemic MR is a more appropriate indicator of procedural success than freedom from reoperation, because many patients with suboptimal results do not undergo reoperation for several reasons such as advanced age, severe LV systolic dysfunction, comorbidities etc. However, patients with postoperative ischemic MR greater than 2+ have a worse survival than those without significant ischemic MR recurrence Citation13.
In patients without functional recovery after CABG, improvement of ischemic MR by annuloplasty resulted in favorable LV remodeling. These results suggest that undersized simple annuloplasty may be clinically preferable, because it has favorable effects on LV remodeling, as well as a lower operative risk compared with more complex procedures Citation17.
However, recent studies in several centers have demonstrated that MR can persist or recur after annuloplasty Citation18, Citation19. Remodeling is progressive Citation20, Citation21, so that initial annular compensation for ventricular dilatation may not be durable. According to the data of our study LV remodeling continues in 30.3% of patients in one year following mitral repair.
Baseline LV remodeling plays a key role in the likelihood of reverse remodeling and subsequent favorable long-term outcome Citation13. We have found that patients with further postoperative LV remodeling present with significantly higher preoperative LVEDD.
Our earlier study has proved that in patients undergoing combined CABG and MV repair, severe LV systolic dysfunction and restrictive LV diastolic filling pattern are important preoperative markers of high perioperative mortality rate, further negative remodeling of LV, and progression of mitral regurgitation late after MV repair Citation22. Other studies have revealed also, that interpapillary muscle distance, as reliable index of dysfunctional subvalvular apparatus in patients with ischemic MR, can predict late postrepair MR and indicate the need for procedure complementary to annuloplasty Citation23.
The main goal of reducing the likelihood of ischemic MR recurrence is to achieve an optimal repair in the operating room with no residual or trivial ischemic MR. The absence of residual ischemic MR is crucial in preventing its deterioration during follow-up because the presence of mild ischemic MR can contribute to continued LV remodeling resulting in a vicious cycle in which IMR begets more ischemic MR Citation13. Our data suggest that the postoperative remodeling could be associated with worse early postoperative results as higher early postoperative MR grade was found in further LV remodeling group.
Further LV remodeling is associated with significantly higher mean MR grade, mitral annulus sagital diameter, LA diameter one year after surgery. Otherwise these variables could themselves contribute to extensive LV remodeling after mitral repair.
The degree of regional and global myocardial scarring is correlated with the severity of MR due to the resultant geometric and functional changes, and each region of scar (inferior-posterior and anterior-lateral) has an independent impact on MR. Once ischemic MR starts, end-diastolic LV volume and wall stress increase side by side with preload causing more LV systolic dysfunction, which in turn results in further papillary muscle displacement and leaflet tenting. Therefore, ischemic MR begets ischemic MR in a self-perpetuating manner Citation13. According to our data, LV wall motion score index and congestive heart failure functional class (NYHA) appears higher with no significant improvement in LV EF in LV remodeling group one year after surgery. These findings suggest that LV remodeling after MV repair is associated with worse functional status of the patient.
According to our study predictive factors for further LV remodeling after ischemic mitral repair 1 year after surgery are: preoperative LVEDDi, preoperative LVESDi, TR grade before surgery, and early postoperative MR grade.
LV reverse remodeling and recurrent MR occurring after CABG and MV repair indicate that LV remodeling might be a progressive ventricular problem that cannot be treated by annuloplasty Citation24, because it reduces tethering only at the annular and not at the ventricular end. Progressive ventricular remodeling in ischemic heart disease can therefore increase tethering and worsen MR despite initial reduction. The ability of annuloplasty to compensate for ventricular tethering can then be overwhelmed by further ventricular remodeling Citation25. Thus, more rational surgical solution in this patient population could be operative techniques targeting LV geometry restoration and preventing further ischemic distortion of LV and MV apparatus Citation26.
Limitations
The present study was neither prospective nor randomized, which might represent a major limitation. It should also be noted that the preoperative evaluation of viability might help to distinguish between irreversible and reversible LV wall motion abnormalities, and may indicate which patients would benefit most from this approach.
Conclusions
LV remodeling continues in 30.3% of patients in one year following ischemic mitral repair.
Predictive factors for further LV remodeling after ischemic mitral 1 year after surgery are as following: preoperative LVEDDi, preoperative LVESDi, TR grade before surgery, and early postoperative MR grade.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation 1997; 96: 827–33
- Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, et al. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation 2005; 111: 295–301
- Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103: 1759–64
- Adler DS, Goldman L, O'Neil A, Cook EF, Mudge GH, Shemin RJ, et al. Long term survival of more than 2000 patients after coronary artery by pass grafting. Am J Cardiol 1986; 58: 195–202
- Lamas GA, Mitchell GF, Flaker GC, Smith SC, Gersh BJ., Basta L, et al. Clinical significance of mitral regurgitation after acute myocardial infarction: Survival and ventricular enlargement investigators. Circulation 1997; 96: 827–33
- Enriquez-Sarano M. Timing of mitral valve surgery. Heart 2002; 87: 79–85
- Christenson JT, Simonet F, Bloch A, Maurice J, Velebit V, Schmuziger M. Should a mild to moderate ischemic mitral regurgitation in patients with poor left ventricular function be repaired or not?. J Heart Valve Dis 1995; 4: 484–9
- Arcidi JM, Hebeler RF, Carver JM, Jones EL, Hatcher CR, Jr, Guyton RA. Treatment of moderate mitral regurgitation and coronary artery disease by coronary bypass alone. J Thorac Cardiovasc Surg 1988; 95: 951–9
- Ryden T, Hanssen OB, Wognsen GB, Nilsson F, Svensson S, Jeppson A. The importance of grade 2 ischemic mitral regurgitation in coronary artery by pass grafting. Eur J Cardiothorac Surg 2001; 20: 276–81
- Di Donato M, Frigiola A, Menicanti L, Boghdabi A, Montericcio V, Ranucci M. Moderate ischemic mitral regurgitation and coronary artery bypass surgery: Effect of mitral repair on clinical outcome. J Heart Valve Dis 2003; 12: 272–9
- Harris KM, Sundt TM, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve?. Ann Thorac Surg 2002; 74: 1468–75
- Vaskelyte J, Stoskute N, Ereminiene E, Zaliunas R, Benetis R, Sirvinskas E. The impact of unrepaired versus repaired mitral regurgitation on functional status of patients with ischemic cardiomyopathy at one year after coronary artery bypass grafting. J Heart Valve Dis 2006; 15: 747–54
- Agricola E, Oppizzi M, Pisani M, Meris A, Maisano F, Margonato A. Ischemic mitral regurgitation: Mechanisms and echocardiographic classification. Eur J Echocardiogr 2008; 9: 207–21
- Agricola E, Oppizzi M, Maisano F, De Bonis M, Schinkel AFL, Torracca L, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 2004; 5: 326–34
- Carpentier A. Cardiac valve surgery – the “French correction.”. J Thorac Cardiovasc Surg 1983; 86: 323–37
- McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004; 128: 916–24
- Ocura H, Takada Y, Kubo T, Asawa K, Tagushi H, Toda I, et al. Functional mitral regurgitation predicts prognosis independent of left ventricular systolic and diastolic indices in patients with ischemic heart disease. J Am Soc Echocardiogr 2008; 21: 355–60
- Jebara VA, Dervanian P, Acar C, Grare P, Mihaileanu S, Chauvaud S, et al Mitral valve repair using Carpentier techniques in patients more than 70 years old. Early and late results. Circulation. 1992;86((5 Suppl)):II53–II59.
- Spencer FC, Galloway AC, Grossi EA, Ribakove GH, Delianides J, Baumann FG, et al. Recent developments and evolving techniques of mitral valve reconstruction. Ann Thorac Surg 1998; 65: 307–13
- Hung J, Handschumacher MD, Rudski L, Chow CM, Guerrero JL, Levine RA. Persistence of ischemic mitral regurgitation despite annular ring reduction: Mechanistic insights from 3D echocardiography. Circulation 1999; 100: I73
- Udelson JE, Patten RD, Konstam MA. New concepts in post-infarction ventricular remodeling. Rev Cardiovasc Med 2003; 4(Suppl3)S3–S12
- Ereminiene E, Vaskelyte J, Benetis R, Stoskute N. Ischemic mitral valve repair: Predictive significance of restrictive left ventricular diastolic filling. Echocardiography 2005; 22: 217–224
- Roshanali F, Mandegar MH, Yousefnia MA, Ravatzadeh H, Alaeddini F. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg 2007; 84: 745–9
- Kang DH, Kim MJ, Kang SJ, Song JM, Song H, Hong MK, et al. Mitral valve repair versus revascularisation alone in the treatment of ischemic mitral regurgitation. Circulation 2006; 114: I499–I503
- Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen B, Duran CM, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty. continued LV remodeling as a moving target. Circulation 2004; 110: II85–II90
- Vaskelyte J, Ereminiene E, Benetis R, Zaliunas R, Sirvinskas E. Ischemic mitral valve repair: Correlations between the mechanisms of mitral regurgitation and left ventricular function prior to and following surgery. Scand Cardiovasc J 2005; 39: 182–8