Abstract
Objectives. Angiographic late lumen loss measured 6 to 9 month after bare metal stent implantation in the coronary arteries is a validated restenosis parameter. Design. We performed a second angiographic follow-up after 4 years in event free survivors from the DANSTENT trial cohort. Results. Quantitative comparison of paired coronary angiograms at 6 months and 4 years showed a reduction of late loss from 0.68±0.52mm to 0.42 (±0.52) (mean difference 0.26 (0.17 to 0.36), p<0.0001). Minimal instent lumen diameter had increased from 2.39±0.62mm to 2.64±0.56mm (mean difference: −0.24mm, 95% confidence interval: −0.34mm to −0.14mm, p<0.0001). Instent diameter stenosis decreased from 24.8±14.2% to 18.6±9.3% (mean difference 6.16%, 95% confidence interval: 2.82 to 9.48%, p=0.0006). This observed spontaneous decrease of instent restenosis corresponds to a 19% increase of minimal cross-sectional vessel area and a 39% reduction of the binary restenosis rate over time. Conclusions. Instent late lumen loss in bare metal stents decreases spontaneously over time. Maturation of early hyperplastic tissue reaction after stent implantation with subsequent thinning of fibrotic tissue might explain this phenomenon.
Instent late lumen loss in coronary stents, measured by angiography, is frequently used as a surrogate endpoint for clinical restenosis. Instent restenosis is seen in 10 to 50% of cases Citation1–4. Initial studies of the bare metal stent showed that neointimal hyperplasia mainly developed during the first few months after stent implantation, and thereafter remained unchanged or decreased Citation5–8. Newer stents, which elute drugs, have been proven to reduce instent restenosis, in a variety of lesion subsets Citation9–13. However, conventional bare metal stents are still in frequent use, and to improve the understanding of the clinical relevance of the commonly used 6 to 9 months restenosis parameters in bare metal stents, we decided to study the spontaneous development of angiographic and clinical instent restenosis parameters in the very long term.
Methods
Patients, inclusion and exclusion criteria
The randomised DANSTENT trial compared first and second generation bare metal stents in de novo lesions Citation14, Citation15. All survivors from the DANSTENT trial, living in Copenhagen, were invited to participate in a second clinical and angiographic follow-up. Exclusion criteria for angiography were target lesion revascularisation, and reduced renal function with a plasma creatinine >150 micromoles/l or intolerance to contrast media.
The study protocol was approved by the local scientific ethics committee (KF01-031/02) and conformed to the Declaration of Helsinki. All patients gave written informed consent to participate.
Quantitative coronary angiography
All coronary angiograms for quantitative coronary angiography analysis were acquired after intracoronary injection of 0.2 mg of glyceryl nitrate in identical views according to guidelines as compared to angiograms at the initial stent procedure and the 6 months follow-up. Angiograms were stored in DICOM on compact discs. The independent core laboratory (Bio-Imaging Technologies B.V. Leiden), that earlier had performed the analysis of the procedural and 6 months follow-up angiograms, also performed the present second follow-up standardized quantitative coronary angiography analyses using a validated algorithm with the angiography catheter as scaling device (QCA-CMS version 4, MEDIS medical imaging systems, the Netherlands) Citation16.
Clinical follow-up
Any death was identified in the Central Person-Identity Registry of Denmark. All study participants had their admittances to hospitals in the Copenhagen Hospital Cooperation and all other hospitals in the Eastern Denmark identified in a centralized hospital register and doctor's letters were acquired when appropriate. Patients, who accepted participation in the angiographic follow-up, had a complete in-hospital clinical work-up. Major adverse cardiac events including causes of deaths were identified by combining information from the databases, doctor's letters and, when available the in-hospital work-up. A myocardial infarction was defined as the development of new Q-waves in a 12-lead electrocardiogram and/or a significant rise in myocardium specific enzymes not related to an angiographically successful percutaneous intervention. Target segment revascularisation occurred when repeat angioplasty or coronary bypass surgery was considered necessary due to restenosis in relation to the vessel segment of the study stent. Also, non-target vessel revascularisations were registered.
Statistical analyses
Categorical variables are presented as their percentage of the total number in the group and analysed using the χ2 or Fisher's exact test. Discrete and continuous variables are presented with their means and standard deviations. Data were tested for normal distribution before use of t-tests. Independent groups t-tests, and when appropriate, paired groups t-tests, were used for comparisons between groups in the analysis of angiographic data, except for the subgroup analysis of patients with 6 months diameter stenosis ≥40%, where we used Wilcoxon signed rank test. A Kaplan-Meier analysis was used to present the cumulative distribution of major adverse events. All data were analysed using SPSS statistic analysis program (SPSS Version 11.0, SPSS Inc, Chicago, IL, US). All tests off significance were two-tailed, and only a p-value <0.05 was considered significant.
Results
Patients
Four hundred and twenty four patients were enrolled in the randomised DANSTENT trial of coronary lesions that could be covered with a single 15 or 16 mm bare metal stents of the first or second generation, as previously described Citation14, Citation15. Of the 424 patients, 109 were living in the Copenhagen area and all patients from this area were invited to participate in the present late follow-up study. One patient refused any participation and was excluded from further analysis. The remaining 108 patients were evaluated 4.3 (±1) years after the initial coronary stent implantation. Ten patients had died (3 of cardiac and 7 of non-cardiac causes). Eighteen patients had undergone either percutaneous coronary revascularisation Citation12 or bypass surgery within the first year after stent implantation. The remaining 80 patients were asked to participate in a second angiographic follow-up and 52 (65%) accepted participation. There were more patients with non-target vessel PCI or myocardial infarction among the patients with angiographic follow-up when compared with those without (13 vs. 7, p=0.049). Patients with 4 years angiogram were older than those without 4 years angiogram, otherwise there were no differences in baseline characteristics between patients with both a 6 months and 4 years angiogram, and those with clinical follow-up, only ().
Table I. Baseline characteristics
Clinical follow-up
In the entire 4.3 (± 1.0) years follow-up period fifty of the 108 patients had one or more major adverse events. We performed percutaneous target segment revascularisation in twelve patients during the first year. Other first major adverse events were cardiac death Citation5, other death Citation7, large myocardial infarction Citation6, coronary bypass operation Citation6 and percutaneous non-target segment interventions Citation14. Major adverse events in patients with and without paired 6 months and 4 years angiograms are depicted in . Re-interventions due to symptoms caused by an aggressive restenosis process in the first few months after stent implantation were infrequent. The majority of re-interventions in the target segments were performed percutaneously per-procedure in relation to the 6 months follow-up angiography. After the first 6 months patients' event-free survival rate shows a slow and steady decrease resulting in a 4-year event free survival of less than 60%. The Kaplan-Meier distribution of the time to the patients' first major adverse event is depicted in .
Figure 1. Kaplan Meier distribution of major adverse events Any event=the composite of death (cardiac and non-cardiac), coronary artery bypass surgery, any percutaneous coronary intervention (target segment and non-target segment) and myocardial infarction.
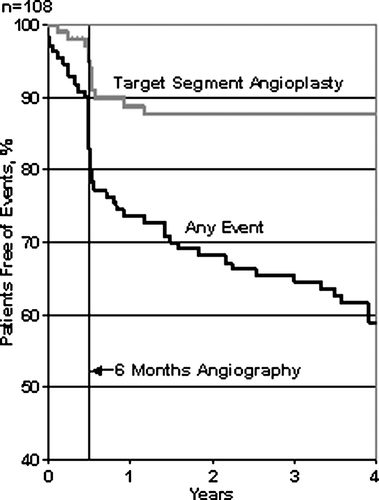
Table II. Number of major adverse events in patients with and without paired 6 months and 4 years angiograms
Angiographic follow-up
Fifty-two patients had 4 years angiographic follow-up, four patients were excluded from the paired angiogram comparison by the core lab due to vessel overlap and/or poor contours and/or foreshortening. Four patients had irretrievable 6 months investigations.
There were significant differences in angiographic baseline parameters between the 44 patients with paired angiograms after 6 month and 4 years follow-up, when compared to patients without angiographic long term follow-up (). In the 44 patients with both angiographic 6 months and 4 years angiograms, we found a highly significant improvement over time in the angiographic restenosis parameters, corresponding to a 19% increase in minimal vessel cross-sectional area ().
Table III. Angiographic baseline data in patients with and without paired 6 months and 4 years angiogram
Table IV. Angiographic results in patients with 4 years follow-up
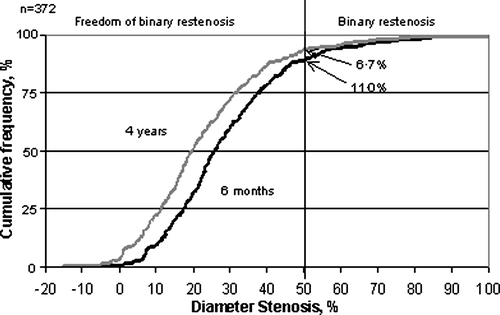
To calculate the expected long-term improvement in binary (>50%) restenosis, we constructed the cumulative distribution curve of vessel diameter stenoses at angiograhic follow-up in the first 6 months. We then for each patient-measure subtracted the average long-term 6.1% reduction of the diameter stenosis to get the expected 4 years cumulative distribution curve of restenosis. This calculation showed an expected binary restenosis after 4 years of 6.7%, which corresponds to a 39% reduction of the binary restenosis rate ().
Figure 2. The expected binary restenosis rate The curve to the right is the cumulative distribution of diameter stenosis in all patients in the DANSTENT study Citation10. In the curve to the left, the expected 4 years cumulative distribution of diameter stenosis, is calculated by subtracting the expected late restenosis regression of 6.15% for each patient. The binary restenosis rates (% of patients with ≥50% diameter stenosis) at 6 months follow-up and at 4 years follow-up are indicated with the arrows
There were no differences in restenosis measures between first and second generation stents at the time of 4 years follow-up (minimal lumen diameter: 2.70±0.66mm vs. 2.60±0.55mm [mean difference 0.10mm, 95% confidence interval −0.25mm to 0.45mm, p=0.57] and diameter stenosis: 18.7±10.8% vs. 18.4±7.0% [mean difference 0.3%, 95% confidence interval −5.0 to 5.6, p=0.91] for the Palmaz-Schatz [n=24], and the NIR stents [n=24], respectively).
In a subgroup analysis we identified a group of patients with borderline significant instent restenosis (≥40%; n=5). These patients had not had re-intervention related to the 6 months follow-up angiography despite a mean diameter stenosis of 52.4±13.3% and a mean minimal lumen diameter of 1.58±0.56mm. At 4 years follow-up, this group of patients had had a spontaneous increase in minimal lumen diameter to 2.28±0.64 (p=0.04), resulting in a reduction of the mean diameter stenosis to 27.6±16.3% (p=0.04).
Discussion
The main finding is that patients who have received a bare metal stent and who have not been revascularised in relation to a 6 months angiographic follow-up show a highly significant regression of angiographic late loss between 6 months and 4 years follow-up. These findings confirm the findings of Kimura et al. Citation5. In their study, late regression of instent restenosis was found between one and three years follow-up. Though, they studied the first generation balloon expandable Palmaz-Schatz stent, only, and they did not use an independent core laboratory for the evaluation of the angiograms. Also, Kimura et al. used low balloon pressures at stent implantation, when compared to our study (9.7±2.1 atmospheres and 15.0±3.0 atmospheres, respectively). Low-pressure implantation of balloon expandable stents is now considered obsolete, as higher pressure has been found to facilitate stent expansion and apposition to the vessel wall and to reduce the risk of subacute stent thrombosis Citation8. Our results seem to demonstrate that late restenosis regression might occur, also with the currently used stent deployment technique.
The patients in the present study were initially randomised to receive either the Palmaz-Schatz stent or the second generation NIR-type of stent Citation15. Regression in late loss was similar in the two types of stents. Therefore, it seems, as late instent restenosis regression is a general phenomenon, which is not limited to the first generation of stents.
Intracoronary late regression of instent restenosis has been demonstrated in the canine model and the mechanism seems to be fibrotic maturation of the early hyperplastic tissue reaction with subsequent and successive thinning of fibrotic tissue Citation17, Citation18. Late thinning of fibrotic tissue is well known also from the human skin or other organs, and neointimal retraction has been shown in a postmortem study of human coronary arteries Citation19, Citation20.
The present study suggests a substantial, 39%, late reduction of binary restenosis in bare metal stents (), and it might be speculated that by similar mechanisms the late loss and restenosis in drug eluting stents might decrease in the very long term. In fact, late loss has independent of stent type or vessel size monotonically been found related to restenosis Citation21.
From well conducted studies, it is well known that Sirolimus and Paclitaxel eluting stents, in the short-, intermediate- and long-term, reduce angiographic late loss and angiography-related operator-decided re-interventions in simple as well as in complex coronary artery lesions, when compared to bare metal stents Citation12, Citation13, Citation22, Citation23. Based on the findings by Kimura et al. and the present study, one might consider that comparisons between bare metal stents and Sirolimus and Paclitaxel and other drug eluting stents might have had a different outcome, if they had been performed years after stent implantation instead of after 6 to 9 months.
In the present study, as in other studies, the majority of re-interventions in the target segments were performed percutaneously per-procedure in relation to the 6 months follow-up angiography (). To overcome the problem of investigator bias in relation to per-procedure re-interventions at the 6 to 9 months follow-up, a clinical event committee might be useful. This committee should preferably judge the appropriateness of intervention before the intervention takes place. However, such a study design is uncommon Citation9–15, Citation22, Citation23.
It might be considered a limitation of the present study that results are based on a subgroup of 44 patients of 108. Since measures of changes in stenosis are likely to fall in a Gaussian distribution, the exclusion of patients who suffered a major adverse event probably have left a skewed population with favourable angiographic changes. Furthermore, the included lesions were de novo, relatively short and in native arteries, and the studied patient cohort was small. Therefore interpretation should be cautious and generalization to other lesion types is not warranted. Further studies are necessary to clarify the possible clinical importance of neointimal retraction. However, in view of the degree of the late loss regression we did observe in patients with paired angiograms, it might be of interest to further study so-called watchful waiting as an alternative to re-intervention in cases of borderline significant angiographic instent restenosis at a 6 months follow-up, in particular, when patient's symptoms are moderate and the appropriateness of angioplasty is not supported by measurements showing reductions in the coronary flow reserve Citation24.
Acknowledgements
The study was supported by grants funded from the Research Foundation of Rigshospitalet, Copenhagen, Denmark. The sponsor has had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the manuscript.
References
- Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994; 331: 489–95
- Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994; 331: 496–501
- de Jaegere P, Mudra H, Figulla H, Almagor Y, Doucet S, Penn I, et al. IVUS guided optimised stent deployment: Immediate and 6 months clinical angiographic results from Multicenter Ultrasound Stenting in Coronaries study (MUSIC). Eur Heart J. 1998; 19: 1214–23
- Lansky AJ, Roubin GS, O'Shaughnessy CD, Moore PB, Dean LS, Raizner AE, et al. Randomized comparison of GR-II stent and Palmaz-Schatz stent for elective treatment of coronary stenoses. Circulation. 2000; 102: 1364–8
- Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med. 1996; 334: 561–6
- Laham RJ, Carrozza JP, Berger C, Cohen DJ, Kuntz RE, Baim DS. Long-term (4- to 6-year) outcome of Palmaz-Schatz stenting: Paucity of late clinical stent-related problems. J Am Coll Cardiol. 1996; 28: 820–6
- Kiemeneij F, Serruys PW, Macaya C, Rutsch W, Heyndrickx G, Albertsson P, et al. Continued benefit of coronary stenting versus balloon angioplasty: Five-year clinical follow-up of Benestent-I trial. J Am Coll Cardiol. 2001; 37: 1598–603
- Nakamura S, Colombo A, Gaglione A, Almagor Y, Goldberg SL, Maiello L, et al. Intracoronary ultrasound observations during stent implantation. Circulation. 1994; 89: 2026–34
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. Randomized study with the sirolimus-coated Bx Velocity balloon-expandable stent in the treatment of patients with de nove native coronary artery lesions: A randomised comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346: 1773–80
- Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: A randomized controlled trial. JAMA. 2005; 294: 1215–23
- Kelbaek H, Helqvist S, Thuesen L, Kløvgaard L, Jørgensen E, Saunamæki K, et al. Long-term outcome in patients treated with sirolimus-eluting stents in complex coronary artery lesions: 3-year results of the SCANDSTENT (Stenting Coronary Arteries in Non-Stress/Benestent Disease) trial. J Am Coll Cardiol. 2008; 51: 2011–6
- Kelbæk H, Thuesen L, Helqvist S, Clemmensen P, Kløvgaard L, Kaltoft A, et al. Drug-eluting versus bare-metal stents in patients with ST-segment elevation myocardial infarction. Circulation. in press.
- Jørgensen E, Ripa RS, Helqvist S, Wang Y, Johnsen HE, Grande P, et al. Instent neo-intimal hyperplasia after stem cell mobilization by granulocyte-colony stimulating factor. Preliminary intracoronary ultrasound results from a double-blind randomized placebo-controlled study of patients treated with percutaneous coronary intervention for ST-Elevation Myocardial Infarction (STEMMI Trial). Int J Cardiol. 2006; 111: 174–7
- Jørgensen E, Kelbæk H, Helqvist S, Jensen GV, Saunamäki K, Kastrup J, et al. Predictors of coronary in-stent restenosis. Importance of angiotensin converting enzyme gene polymorphism and treatment with angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2001; 38: 1434–9
- Jørgensen E, Kelbæk H, Helqvist S, Jensen GV, Saunamäki K, Kastrup J, et al. Low restenosis rate of the NIR coronary stent. Results of the DANSTENT study: A randomised trial comparing a first with a second generation stent. Am Heart J. 2003; 145: e5
- Van Weert AWM, Lesperance J, Reiber JHC. Standardization of central off-line quantitative image analysis: Implications from experiences with quantitative coronary angiography. Heart Drug. 2001; 1: 44–51
- Schatz RA, Palmaz JC, Tio FO, Garcia F, Garcia O, Reuter SR. Balloon expandable stents in the adult dog. Circulation. 1987; 76: 450–7
- Robinson KA, Roubin GS, 3rd, King SB. Long-term intracoronary stent placement: Arteriographic and histologic results after 7 years in a dog model. Cathet Cardiovasc Diagn. 1996; 38: 32–7
- Nobuyoshi M, Kimura T, Ohishi H, Horiuchi H, Nosaka H, Hamasaki N, et al. Restenosis after percutaneous transluminal coronary angioplasty: Pathologic observations in 20 patients. J Am Coll Cardiol. 1991; 17: 433–9
- Farb A, Kolodgie FD, Hwang H-D, Burke AP, Tefera K, Weber DK, et al. Extracellular matrix changes in the stented human coronary arteries. Circulation. 2004; 110: 940–7
- Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation. 2005; 111: 3435–42
- Windecker S, Remondino A, Eberli FR, Jüni P, Räber L, Wenaweser P, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Eng J Med. 2005; 353: 653–62
- Morice MC, Colombo A, Meier B, Serruys P, Tanburino C, Guagliami G, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions. The REALITY Trial: A randomized controlled trial. JAMA. 2006; 295: 895–904
- Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation. 2001; 103: 2928–34