Abstract
Objectives. Training improves exercise capacity in patients with heart failure (CHF) but most evidence is on selected younger patients with systolic CHF. Design. All patients diagnosed with CHF over 3 years were screened for inclusion and exclusion criteria. Fifty two patients with systolic CHF (LVEF<45, NYHA II-III) received supervised exercise training twice weekly for 8 weeks. Results. Mean age was 68.2 (+/−SD 11.3) years. Despite marked improvements in physical fitness (workload, 6 minute walk test, incremental shuttle walk test and sit to stand test), there were no changes in serological markers of glycemic control (glucose, insulin, glycerol, free fatty acids, HbA1c), inflammation and endothelial function (hsCRP, orosomucoid, interleukin 6, TNF-alpha, urine-orosomucoid and -albumin/creatinin), lipid metabolism, NT-proBNP or other regulatory hormones (cortisol, epinephrine and IGF-1). There were no changes in quality of life. Conclusions. The effect of exercise training in these older CHF-patients was not as impressive as reported in younger and more selected patients. More studies on the efficiency of exercise training that reflect the age- and co-morbidity of the majority of CHF-patients are needed.
A large number of controlled trials have shown that patients with chronic heart failure (CHF) benefit physically from exercise training and this in turn improves their quality of life. Most of these studies have however been conducted on highly selected groups of primarily younger males with systolic heart failure and no or only slight co-morbidity, e.g. in a Cochrane review comprising 29 studies only three studies included patients with a mean age above 65 Citation[1]. This is in discrepancy with demographic data reporting that 88% of patients with CHF are above 65 years of age and 49% above 80 at the time of diagnosis Citation[2] and raises the question whether the impressive results of exercise training in reported studies are transferable to a true clinical population with high age, co-morbidity and probably poorer adherence.
The aim of this study was to evaluate feasibility and effect of an 8 weeks training program in a clinical setting consecutively including all eligible patients with CHF. Further, although the beneficial effect of exercise training in CHF on exercise capacity and physical functioning tests is unequivocal, effects of training on markers of inflammation, glycemic control and endothelial damage are not yet clarified.
Methods
Patient selection
All patients with a diagnosis of CHF referred to an outpatient clinic or discharged from hospital with a diagnosis of CHF from January 1, 2002 to January 31, 2005 at Amager University Hospital were consecutively screened for inclusion (). Inclusion criteria were: left ventricular ejection fraction (LVEF) ≤ 45% assessed by a recent echocardiography (<6months); New York Heart Association (NYHA) functional class II–IV; optimal medical treatment including maximal tolerated dosage of beta-blocker, ACE inhibitor or ARB; and compensated heart failure for at least 3 weeks in agreement with guidelines Citation[3]. Exclusion criteria were: Severe valvular heart disease; musculoskeletal disability; other chronic disease with expected life span < 1 year; and ischemia or angina at low strain (≤45 watts) or malignant cardiac arrhythmias (>3 consecutive VES, VT or SVT with ventricular action of >150 bpm) during baseline cycle ergometer testing.
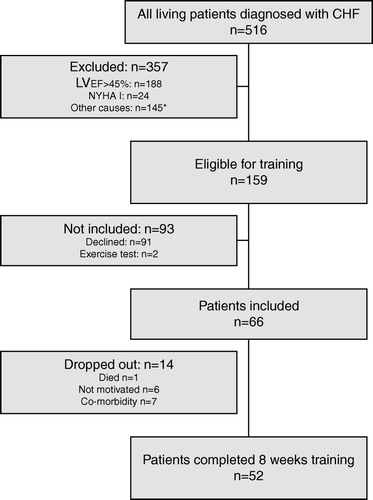
Figure 1. Flowchart of patient selection. All patients alive with a diagnosis of CHF referred to an outpatient clinic or discharged from hospital form January 1, 2002 to January 31, 2005. *Other causes were mainly musculoskeletal disability impeding exercise training and/or transportation (n = 61), dementia (n = 32), valvular heart disease (n = 11), co-morbidity with limited life-span (n = 10), other reasons (n = 19), could not be contacted (n = 12).
Patient assessment
Background data included medical history, socioeconomic data (level of education, cohabitation and employment status) and a questionnaire on cardiovascular risk factors and symptoms. Assessment at baseline and 8 weeks consisted of exercise testing, functional tests, anthropometric data, the Minnesota “living with heart failure” quality of life questionnaire and serological markers.
Most studies of exercise training in CHF have used directly measured VO2max as main outcome, while some have used maximum work capacity, exercise duration or the 6-min walk-test (6MWT). Meta-analyses have shown a homogeneous beneficial effect of exercise training in all of these modalities Citation[1]. We used maximal symptom limited cycle ergometer test with a standardised protocol of a workload of 25 watts increasing the intensity every 2 min with 10 watts until physical exhaustion. Since patients were encouraged to home-based walking with calibration of walking speed, we included the incremental shuttle walk test (ISWT), which has good reproducibility and close correlation to VO2max in CHF-patients and also predicts event-free survival in patients with CHF Citation4–7. Muscle strength was measured by a sit-to-stand test (STS) previously validated in elderly persons Citation[8]. Patients were instructed in getting up and sitting back down as many times as possible within 30 s using the chairs armrests. Quality of life was assessed using the Minnesota living with heart failure questionnaire with subscales of physical and emotional dimensions Citation[9]. Missing data on questions regarding work limitations and sexual activity were regarded as irrelevant and coded as 0 at both examinations.
Serological markers
Fasting venous blood was sampled at baseline and 8 weeks and analysed for metabolic markers (HbA1c, glucose, insulin, glycerol and free fatty acids), markers of inflammation and endothelial function (hsCRP, orosomucoid, interleukin 6 (IL-6), TNF-alpha, urine-orosomucoid and urine-albumin/creatinin) and other serological markers (NT-proBNP, homocystein, HDL-cholesterol, total cholesterol, LDL-cholesterol, triglycerides, cortisol, epinephrine and insulin like growth factor 1 (IGF-1)). (For details on analyses please see appendix).
Exercise training
Danish national guidelines recommend exercise training in stable patients within weeks following optimal medical treatment and thus we did not include a control group but instead patients served as their own control. The supervised training program was performed twice weekly for 8 weeks in a hospital based rehabilitation facility. Each session comprised a 15 – 20 min warm-up period followed by a circuit-training program with four 6-minutes series of aerobic training (walking, cycling, step machine and step board) and two posts of resistance endurance exercises (leg press and exercises with rubber-bands for quadriceps, gluteus/hamstring region and arms; 3 sets of 20 repetitions with each arm/leg). Training intensity was initially aimed at 70 – 80% of maximal heart rate measured during cycle ergometer but this was later abandoned because of narrow heart rate reserve in most patients, atrial fibrillation and other problems. Instead, using the ISWT at baseline testing, each patient's walking speed was adjusted to achieve 70 – 80% of peak oxygen consumption. The patients were instructed to exercise at this intensity corresponding to 4 – 5 on the modified Borg scale (range 0 (no breathlessness at all) to 10 (maximal breathlessness)). Training intensity and resistance was continually adjusted to the desired level. Following each circuit, 5 – 10 min of stretching and debriefing was performed. The total time used for each group-based exercise training session was between 1 and 1.5 hours.
Patients were encouraged to walk or do other physical training for 20 – 30 min. each day at home at a similar level of exertion and were given rubber bands for resistance training exercises at home on a daily basis.
Statistical analysis
Groups were compared with 2-sided χ2 statistics, unpaired t-tests for normally distributed data and rank-sum tests for data not normally distributed. Changes within groups were compared by paired t-test or signed rank test. Predictors of adherence were determined by logistic regression analyses and predictors of change in functional tests by linear regression. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed in Stata 10.0.
Results
Of the 516 patients alive diagnosed with CHF, 159 (31%) had systolic heart failure and were eligible for training according to guidelines Citation[3], 66 (13%) wished to participate and 52 patients completed the 8 weeks exercise training program ().
Baseline characteristics are shown in . Mean age of completers was 68.2 years (range 30 – 86). In 80% the aetiology for CHF was IHD. Medication was optimal with 85% on beta-blockers and 98% on ACE inhibitors/ARB. None of the patients had CRT. The 14 patients that dropped out of the study differed from completers only in BMI (). A logistic regression to determine which covariates were independently associated with drop-out confirmed that only BMI was a significant predictor whereas age, gender, disease severity as measured by LVEF, NT-proBNP and NYHA class, co-morbidity, baseline exercise capacity and socioeconomic factors did not affect drop-out rate. During the study period one person died (not related to exercise training) and five patients died within the following 12 months.
Table I. Baseline characteristics of patients completing 8 weeks training and drop-outs. Values are given as mean (SD) or number (%).
Exercise test data at entry and after 8 weeks training are presented in . The functional tests all improved significantly: for maximal workload, exercise time, 6MWT and ISWT by 5 – 7%, for STS by 13%. The size of the changes was not associated with baseline exercise capacity, measures of disease severity (LVEF, NT-proBNP and NYHA class), age or gender.
Table II. Functional tests before and after 8 weeks exercise training program in 52 patients with systolic heart failure. Values are mean (SD).
The metabolic markers, markers of inflammation and endothelial function and other serological markers did not change from baseline to study end (). There was no difference between before and after values in the total Minnesota living with heart failure score or in the subscales of physical and emotional dimensions ().
Table III. Serological markers before and after 8 weeks exercise training in 52 patients with systolic heart failure. Values are mean (SD).
Table IV. Quality of life score (Minnesota living with heart failure) before and after 8 weeks exercise training program in 52 patients with systolic heart failure. Values are mean (SD).
Discussion
The present study is an attempt to bridge the gap between experiences form studies of highly selected and motivated younger males to the clinical reality of older patients of both sexes with considerable co-morbidity. In these patients with CHF, 8 weeks of supervised exercise training combined with home-based training resulted in improvement in all functional tests whereas no effects in quality of life or serological markers of inflammation, glycemic control, endothelial function, lipid metabolism, NT-proBNP or other regulatory hormones were found.
Demographic data have shown that 88% of CHF-patients are above age 65 Citation[2], yet in 26 of 29 studies in a meta-analysis from 2004 mean age of patients was below 65 years Citation[1], and similar age-distributions were found in later reviews Citation[10], Citation[11]. Thus it is not known whether the impressive results of 15 – 25% improvement in VO2max reported in the majority of studies are applicable to the clinical reality where patients are older. In the present study exercise capacity improved significantly although the size of the improvement was modest and no effect was seen in quality of life. Subgroup analyses of pooled studies indicated poorer effect in patients aged more than 55 years (improvement in peak VO2 1.77 (1.03 – 2.5) versus 2.95 (1.64 – 4.25)), although this difference was insignificant Citation[1]. In exercise training studies of older patients results have also been modest. Supervised training once weekly for 12 weeks in 19 CHF-patients above 75 years (mean age 81) resulted in an increase in 6MWT from 209 to 231 metres (p = 0.012) but no improvement in quality of life Citation[12]. Aerobic and resistance training twice a week for 5 months in 21 CHF-patients (mean age 68) resulted in significant improvement in work-load (88.3 to 95.9 watt, p = 0.007), 6MWT (from 489 to 526, p = 0.001) and muscle strength, but not in quality of life Citation[13]. In the largest study of this age group, the EXERT study (n = 80 in the training group, mean age 64.8 yrs) supervised aerobic and resistance training twice weekly for 3 months was also associated with a modest increase in 6MWT (baseline 434 meters, improved by 22 + /− 5 metres), peak oxygen uptake (10%, p < 0.01) and muscle strength but not in quality of life Citation[14]. Thus results are comparable to other studies of similar age groups and confirm that training effects are probably more difficult to attain in unselected elderly patients with CHF.
We found no changes in markers of inflammation following 8 weeks training. Endothelial damage and inflammatory cytokines are involved in the pathogenesis of CHF Citation[15]. Acute bouts of exercise lead to increases in markers of endothelial damage (e.g. VCAM-1and P-selectin) and pro-inflammatory cytokines (e.g. IL-6, TNF-alpha) but several studies have reported a decrease in at least some cytokines following more prolonged training Citation16–19. However, a study similar to ours in which 18 CHF-patients trained for 8 weeks (mean age 53) found no changes in pro-inflammatory cytokines and markers of endothelial damage Citation[20]. Twelve weeks of endurance training twice weekly in nine patients with CHF (mean age 69) did not change IGF-1, endothelin 1, hsCRP or proBNP Citation[21]. In a recent study of 15 CHF-patients (mean age 57), 20 weeks of exercise training was associated with a significant decline in the inflammatory markers soluble CD40 ligand, P-selectin and total leucocyte count but not in TNF-alpha, VCAM-1 and MCP-1 and none of the changes were associated with changes in exercise capacity Citation[22]. Thus results are mixed. It has been suggested that exercise training mainly reduces local muscular expression of TNF-alpha, IL-1-beta and IL-6 in CHF-patients, which may not be discernable in plasma measurements Citation[18].
The relationship between insulin sensitivity and exercise capacity in healthy subjects and diabetic patients is well known. CHF is associated with increased insulin resistance and exercise training would also be expected to improve glycemic control in this patient group. This has been the subject of a few studies with varying results. One small study (training group: n = 9) found an increase in insulin sensitivity concomitantly with an increase in VO2-max following 5 months of exercise training Citation[23] but in a larger controlled study (training group: n = 36) exercise training four times weekly for 26 weeks had no effect on insulin sensitivity despite significant effect on peak VO2Citation[24]. The present study found no effects on markers of glycemic control (fasting glucose, HbA1c, insulin) perhaps because of the short duration of the study, with particular consequence for HbA1c.
We found no effect of 8 weeks training on quality of life. Although this is disappointing it is not surprising. Most previous studies of endurance training in elderly patients with CHF have reported that effects in physical capacity do not translate to an effect in quality of life Citation12–14.
This study provides important information on exercise training in a clinical setting. Few training studies of heart failure patients have reported the selection procedures that have led to their study population. In the present study only a small fraction of the initial patients diagnosed with heart failure were in fact eligible for participation according to guidelines. Less than half of the eligible patients wished to participate. Similar findings were recently reported from a British study in which only 169 of 1 639 patients followed in a CHF clinic could be included in a home based training study Citation[25]. Extending inclusion criteria to patients with heart failure with preserved LVEF would have resulted in more eligible patients, but since the bulk of evidence on exercise training is based on patients with systolic heart failure and the group of patients with non-systolic heart failure is less well defined, this was beyond the scope of this study. The higher age and mortality – six patients died during 14 months – indicate that these patients were probably more representative of the CHF population than in most previous studies.
Study limitations include the lack of a control group. National guidelines recommend exercise training and we found it unethical not to offer this to all patients. However, it is unlikely that the effects found represent a ‘learning effect’ since previous controlled studies have not shown learning effects in control-groups. The modest effect of exercise may be related to the relatively short duration of exercise intervention. Most effect of training is found within a 6 – 8 weeks period. However, studies have indicated a gain from extended training Citation[1], perhaps particularly in an elderly population in which adherence to training sessions is expected to be lower due to co-morbidity. The modest effect may also be related to the fact that our patients were not very sick from CHF as judged by their 6MWT and NYHA class, but this is not unsimilar to other studies: In the large EXERT study of 181 patients with systolic heart failure mean 6MWT was 428 metres Citation[14]. The average maximal workload of approximately 95 watt found in the present study can be calculated to be equivalent of an estimated VO2max of 15 ml/kg/min, which is similar to the seminal study by Belardinelli Citation[26]. The optimal medical treatment regime of these patients may also play a role. In most of the previous studies patients have not been on optimal treatment according to present day guidelines and it has been suggested that patients not receiving beta-blockers have better effect of exercise training.
In conclusion, this study probably reflects more accurately the response to training of patients with systolic heart failure in a clinical setting than do most other studies with unclear selection mechanisms and younger patient groups. In conjunction with previous studies of elderly CHF-patients these results suggest that the effect of exercise training in elderly CHF-patients is not as impressive as estimated based on studies of younger patients. More studies of exercise training in elderly CHF-patients that reflect the demographics of the disease are needed to determine optimal training protocols in this patient group.
Acknowledgements
We wish to acknowledge the important financial contributions from the Velux Foundation, the Danish Heart Foundation, The Danish Ministry of Health, and the Bispebjerg Research Fund. Declaration of interest: There are no conflicts of interest to be declared.
References
- Rees K, Taylor R, Singh S, Coats A, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev. 2004; 3: CD003331
- Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: A study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998; 98: 2282–9
- Recommendations for exercise training in chronic heart failure patients. Eur Heart J. 2001;22:125–35.
- Morales FJ, Martinez A, Mendez M, Agarrado A, Ortega F, Fernandez-Guerra J, et al. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am Heart J. 1999; 138: 291–8
- Morales FJ, Montemayor T, Martinez A. Shuttle versus six-minute walk test in the prediction of outcome in chronic heart failure. Int J Cardiol. 2000; 76: 101–5
- Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001; 119: 256–70
- Green DJ, Watts K, Rankin S, Wong P, O'Driscoll JG. A comparison of the shuttle and 6 minute walking tests with measured peak oxygen consumption in patients with heart failure. J Sci Med Sport. 2001; 4: 292–300
- Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res. 2005; 19: 717–20
- Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: Reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992; 124: 1017–25
- Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J Am Coll Cardiol. 2007; 49: 2329–36
- van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: A meta-analysis. Eur J Heart Fail. 2006; 8: 841–50
- Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. Eur J Heart Fail. 2000; 2: 65–70
- Jonsdottir S, Andersen KK, Sigurethsson AF, Sigurethsson SB. The effect of physical training in chronic heart failure. Eur J Heart Fail. 2006; 8: 97–101
- McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, et al. Effects of exercise training in patients with heart failure: The Exercise Rehabilitation Trial (EXERT). Am Heart J. 2002; 144: 23–30
- Niebauer J. Effects of exercise training on inflammatory markers in patients with heart failure. Heart Fail Rev. 2008; 13: 39–49
- Adamopoulos S, Parissis J, Kroupis C, Georgiadis M, Karatzas D, Karavolias G, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001; 22: 791–7
- Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, et al. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J. 2002; 23: 1854–60
- Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003; 42: 861–8
- LeMaitre JP, Harris S, Fox KA, Denvir M. Change in circulating cytokines after 2 forms of exercise training in chronic stable heart failure. Am Heart J. 2004; 147: 100–5
- Niebauer J, Clark AL, Webb-Peploe KM, Coats AJ. Exercise training in chronic heart failure: Effects on pro-inflammatory markers. Eur J Heart Fail. 2005; 7: 189–93
- Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007; 115: 3086–94
- Bjornstad HH, Bruvik J, Bjornstad AB, Hjellestad BL, Damas JK, Aukrust P. Exercise training decreases plasma levels of soluble CD40 ligand and P-selectin in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2008; 15: 43–8
- Kemppainen J, Tsuchida H, Stolen K, Karlsson H, Bjornholm M, Heinonen OJ, et al. Insulin signalling and resistance in patients with chronic heart failure. J Physiol. 2003; 550: 305–15
- Sabelis LW, Senden PJ, Te Boekhorst BC, Hulzebos HJ, Van De WA, Van Haeften TW, et al. Does physical training increase insulin sensitivity in chronic heart failure patients?. Clin Sci (Lond) 2004; 106: 459–66
- Jolly K, Tayor RS, Lip GY, Greenfield SM, Davies MK, Davis RC, et al. Home-based exercise rehabilitation in addition to specialist heart failure nurse care: Design, rationale and recruitment to the Birmingham Rehabilitation Uptake Maximisation study for patients with congestive heart failure (BRUM-CHF): A randomised controlled trial. BMC Cardiovasc Disord. 2007; 7: 9
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999; 99: 1173–82
Appendix
Serum HbA1c, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, orosomucoid and creatinin were analysed right away. HbA1c, glucose, high sensitive C-reactive protein (hsCRP), orosomucoid, total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides were measured by a colorimetric/turbidimetric method (Integra, Roche Diagnostics Scandinavia AB, Glostrup, Denmark). Blood for determination of metabolites and hormones was collected in iced tubes and immediately centrifuged at 4°C. Blood for determination of tumor necrosis factor alpha (TNF-alpha), IL-6, hsCRP, free fatty acids (FFA) and glycerol was stabilized with EDTA (1.5 mg·ml blood−1). Blood for determination of insulin, insulin like growth factor 1 (IGF-1) and NT-proBNP was stabilized with 500 Kalikrenin inhibitory units aprotinin (Trasylol) and blood for determination of epinephrine was stabilized with 5 µm EDTA and 4 µm reduced glutathione in 20 µl of 0.6 n sodium hydroxide (ml blood)−1. Until analysis, all plasma samples were stored at −80°C. Insulin was determined with sandwich ELISA performed according to the manufacturer's instructions (DakoCytomatics, Glostrup, Denmark). Plasma epinephrine concentrations were determined by a radioimmunoassay kit (Labor Diagnostika Nord, Nordhorn, Germany). Plasma FFA and glycerol analysis was carried out by an enzyme color assay (Hitachi 612, Roche, Glostrup, Denmark). TNFα and IL-6 were analyzed by flow cell fluorometry (Luminex 100 IS 2.2 system, Luminex, Austin, Texas, USA) using a commercial kit (LINCOplex HSCYTO-60SK KIT, Linco Research, St. Charles, Missouri, USA). IGF-1 was analyzed by ELISA technique (R&D systems Quantikine DG100 assay, Minneapolis, MN, USA). Plasma NT-proBNP analysis was carried out by Electro Chemi Luminescence Technology (Modular, Roche Diagnostics Scandinavia AB, Glostrup, Denmark). Homocystein was measured by fluorescence polarisation immune analysis using the Abbot AxSYM System.