Abstract
Objectives. The Impella® recovery axial-flow system is a mechanical assist system for use in acute heart failure. This retrospective study reports the use of the device at three cardiothoracic units in Sweden. Design. Fifty patients (35 men, mean age 55.8 years, range 26 to 84 years) underwent implantation of 26 Impella®LP 2.5/5.0 (support-time 0.1 to 14 days), 16 Impella®LD (support-time 1 to 7 days) and 8 Impella®RD (support-time 0.1 to 8 days) between 2003 and 2007. Implantation was performed because of postcardiotomy heart failure (surgical group, n=33) or for various states of heart failure in cardiological patients (non-surgical group, n=17). The intention for the treatments was mainly to use the pump as a ”bridge-to-recovery”. Results. Early mortality in the surgical and non-surgical groups was 45% and 23%, respectively. Complications included infection, 36% and right ventricular failure, 28%. Cardiac output and cardiac power output postoperatively were significantly higher among survivors than non-survivors. Conclusions. The Impella® recovery axial-flow system facilitates treatment in acute heart failure. Early intervention in patients with acute heart failure and optimized hemodynamics in the post-implantation period seem to be of importance for long-term survival. Insufficient early response to therapy should urge to consider further treatment options.
The development and use of left ventricular assist devices (LVADs) have increased dramatically over the last decade Citation[1], Citation[2]. The concept of axial flow pumps in the setting of failure-to-wean was already explored in the nineties with the Hemopump Citation3–5. This concept has been further developed with the Impella® system (Impella CardioSystems GmbH, Aachen, Germany) which includes devices for both left and right ventricular support (). This system has been shown to be an effective device for a variety of indications such as cardiogenic shock, postcardiotomy heart failure and right heart failure Citation6–10. The Impella is a short-term device regularly used by surgeons to treat postoperative acute cardiac failure when an intraaortic balloon pump is not sufficient. In the rapidly developing era of interventional cardiology, the Impella has also evoked interest as a catheter-based assist device to be used in the catheter lab Citation[11]. The use of a short-term device in the treatment of acute heart failure allows time to evaluate recovery of heart function as well as provide enough time to decide whether the patient should be bridged to a long-term assist device and/or eventually cardiac transplantation.
Figure 1. Impella assist device for left (upper) and right (lower) ventricular support. (With permission from AbioMed)
This paper reports the outcome for 50 patients treated with the Impella® system at three Swedish cardiothoracic centers in the period between April 2004 and January 2007. The report presents all patients treated, and thus includes implantations for various indications and severities of circulatory compromise.
Material and methods
The Impella® recovery axial-flow system
The Impella® Recover axial-flow pump system for temporary support in acute heart failure has been well described Citation[9], Citation[12]. The Impella system includes three different LVADs; LD, implanted through the aorta (requiring sternotomy), LP 5.0, implanted retrogradely into the left ventricle from the femoral artery and pumping up to 5 liters, LP 2.5, implanted, possibly percutaneously, via the femoral artery and pumping 2.5 liters. The Impella system also contains a right ventricular assist device (RVAD); RD, to be placed from the right atrium to the pulmonary artery (through a sternotomy). Of note, Abiomed has recently withdrawn the RD for unstated reasons and this device is therefore not commercially available at the present time. In Impella, the motor is located in the device itself, and a cable is connected to a console positioned beside the patient. The speed can be set at nine different levels of performance (P1–P9). In Europe the device is approved for short-term use, up to 10 days, (CE-marked).
Patients
Data from all patients treated with the Impella axial flow pump between April 2004 and January 2007 at three Swedish cardiothoracic surgical centers (Uppsala, Linköping and Stockholm) were retrospectively recorded. The study was approved by the Ethical Committee, Dnr M176-08.
A total of 51 patients were treated with Recover LD (n = 16), Recover LP 5.0 (n = 15), Recover LP 2.5 (n = 11) or Recover RD (n = 9). In one patient the RD was inserted during non-successful cardiac resuscitation, and this patient has not been included in the tables and figures, leaving 50 patients for presentation. All patients could be traced giving a 100% follow-up.
For presentation, the patients were divided into two groups: 1) the surgical group (n = 33) i.e. patients who had cardiac surgery prior to device insertion; and 2) the non-surgical group (n = 17) i.e. no cardiac surgery prior to insertion of the Impella pump. includes demographic data. One patient with post-infarction ventricular septal defect received the Impella for cardiogenic shock, and surgery was delayed for 3 days. Although the Impella was used also for 5 days postoperatively, according to our definition, this patient has been included in the non-surgical group. Transesophageal echocardiography (TEE) was used to verify correct positioning of the pump in the LV. The patients were considered responding to the therapy when the hemodynamics were stabilized with improved cardiac output (CO) and lowering of the filling pressures and/or demonstration of an increased mixed venous oxygen saturation (SvO2). Also, effect of therapy demanded a combination of improved echocardiographic movement, small to moderate doses of inotropic support and an acceptable diuresis. The weaning procedure started when all parameters were stable for at least 24 hours. The speed was set to a lower rate and the patient was followed closely for some hours. If signs of deteriorating hemodynamics were observed, the speed rate was increased again and the weaning procedure was postponed for 24 hours. With stable conditions, the pump rate was gradually decreased to performance P1 running for at least 6 hours before removal.
Table I. Demographic data of Impella patients
Definitions
Cardiogenic shock was defined as a hemodynamic state, secondary to heart failure that is unable to meet systemic circulatory demands without supportive measures, except for correction of volume or vascular resistance. A low cardiac output may be sufficient to meet demands in an anesthetized or sedated patient and hence our reliance on markers of adequate circulation, in particular mixed venous oxygen saturation (SvO2) and echocardiographic evaluation, as used opposed to fixed hemodynamic criteria. In the surgical group of patients, cardiogenic shock was evident when weaning from cardiopulmonary bypass was not possible due to deteriorating circulation and increasing filling pressures. In the non-surgical group echocardiographic evidence of left ventricular and/or right ventricular dysfunction associated with the above-mentioned signs of inadequate circulation were used.
Surgical group
Thirty-three patients underwent cardiac surgery (8 coronary artery bypass grafting (CABG), 5 aortic valve replacement (AVR) or mitral valve replacement (MVR), 6 combined CABG and valve procedures, 5 cardiac transplants, 1 infarction-VSD and 8 miscellaneous procedures). At preoperative evaluation, the left ventricular function was considered normal (EF >50%) in seven (21%) patients, slightly reduced (EF 30 – 50%) in one (3%), markedly reduced (EF 20 – 30%) in four (12%) and severely reduced (EF < 20%) in 18 (55%) patients. Left ventricular function was not recorded in three patients (9%). Prior to surgery, eight patients (25%) were on intraaortic balloon counter pulsation (IABP), one (3%) was supported with a LVAD, 11 patients (33%) were on inotropic support and seven patients (21%) were mechanically ventilated. Fifteen of 19 failure-to-wean patients (79%) received the Impella prior to weaning from the CPB. The remaining 14 patient developed their postoperative cardiac failure in the ICU (n = 11), and for three patients the indications were not recorded. All patients received heparin anticoagulation during Impella therapy with an activated clotting time (ACT) goal of 180 seconds.
For the vast majority of the patients the intention for this treatment was a bridge-to-recovery. All Recover RD were used for acute right ventricular failure when weaning from CPB, two of them in combination with LVAD for bridge-to-transplantation.
Non-surgical group
The seventeen patients in this group were suffering from myocarditis (n = 5), ischemic heart disease (IHD) (n = 9), or the Impella was used prophylactically in high-risk PCI (n = 3). For demographics see Table I. All patients were on inotropic support, four patients (29%) were on IABP and eight patients (57%) were mechanically ventilated related to their cardiogenic chock. The three patients treated prophylactically received the Impella LP 2.5.
Data collection and statistics
The patient data were collected retrospectively from the medical records. Hemodynamics were measured preoperatively, and at 6 and 12 hours postoperatively. Pre- and postoperative laboratory data were evaluated, the last values were taken one week postoperatively. Preoperative data included risk factors for heart disease. All causes of morbidity and mechanical failures were recorded. Mortality was recorded at 7 days, 30 days, 3 months and at one year post-implantation. Cardiac power output (CPO) was measured as cardiac output times mean arterial pressure divided by 451 Citation[13].
The samples were analyzed using the software STATISTICA (StatSoft, Inc. 2004, version 7, Tulsa, Ok). When comparing patient groups the subsets of data were not assessed as being normally distributed. Data were analyzed with the nonparametric Mann-Whitney U test, p < 0.05 was considered significant.
Results
Surgical group
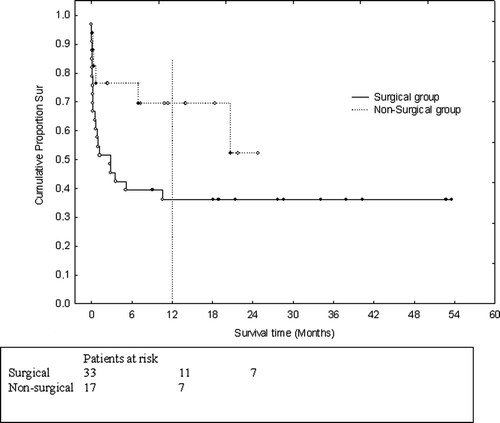
The 30-day mortality was 45% (15/33 patients). Nine of these 15 patients died within one week of their operation. The most common cause of death was multiorgan failure. The 1-year mortality for the surgical patients was 64% (21/33) (). Patients who received Impella RD for right ventricular failure had a 30-day mortality of 75% (6/8), and the one-year mortality for these patients was 87% (7/8). The Impella devices were all together used for a mean of 3.8 days (range, 0.1 to 9 days). The RD was used for a mean of 4.1 days (range, 0.1 to 8 days) and LD/LP for a mean of 3.9 days (range, 0.1 to 9 days). Seventeen patients (52%) were reoperated within 24 hours because of excessive bleeding. Nine patients (27%) were reoperated late in the postoperative course because of bleeding (n = 3), sternal infection (n = 4), late sternal closure (n = 1) and one for an unknown reason. Device failure was recorded in one case at 3 days. This was, according to the manufacturer, due to computer error in the console and the software have since then been updated. No further problems have been noted. The following morbidity was recorded postoperatively: ten patients (30%) had septicemia and 13 patients (39%) required dialysis. Fourteen patients (42%) had transient and pharmacologically treated right ventricular failure, and four of the patients treated with Impella LVAD had transient right ventricular (RV) failure necessitating RV-assist systems. In one patient with heparin-induced thrombocytopenia an increased “purge pressure” was noted. When the device was taken out it was covered with a thin layer of thrombus.
Survivors versus non-survivors in the surgical group
The results are shown in . Survival after 30 days was significantly better for patients with preoperatively placed IABPs (p = 0.01). Cardiac output (CO) at 12 hours and Cardiac Power Output (CPO) at 6 and 12 hours were also significantly higher among survivors. Improved survival postoperatively was indicated by low filling pressures and high mixed venous oxygen saturation (SvO2) after the first 12 hours, but these observations did not reach statistical significance.
Table II. Surgical group.
At one-year follow-up the mortality was 64% (21/33), although one patient had not yet reached the one-year follow-up. The survivors had significantly higher SvO2 (12 hours) and higher CPO at 12 hours. The preoperative use of IABP was a marker of improved survival (p = 0.01).
Non-surgical group
The 30-day mortality in this group was 23% (4/17). Excluding the three patients with LP 2.5 used prophylactically, the 30 day mortality for non-surgical patients with Impellas placed for acute cardiac failure was 21% (3/14). One of the 30 days survivors died during the first year after the treatment. Five of these patients have not yet reached one-year follow-up. The patients in the non-surgical group were significantly younger than the surgical patients. No RV-failure was recorded. Three patients (18%) required dialysis. In this group of patients the left Impella was used for a mean time of 7.3 days (range, 2 to 14 days). One patient was bridged to ECMO after one day and did not survive. One patient was bridged to HeartMate-II after 8 days and later had a cardiac transplant.
Discussion
The need for a mechanical assists device seems to be around 1 – 3% after cardiac surgery Citation[14]. In our material the most frequent indication for the Impella was failure to wean from CPB. By placing a short-term assist system, time can be gained for planning and decision-making for further management of critically ill patients who would otherwise suffer from high morbidity and mortality. In this setting the Impella is easy to use and more powerful than the IABP Citation[15], Citation[16]. In the operating room the LD Impella can be easily placed during sternotomy, even though the trend is to use the LP via the groin also in this situation, avoiding the resternotomy for removing the device. Removal from the groin can be done in the ICU.
The mortality rate was lower in the non-surgical group compared to the surgical group. The non-surgical group consisted of patients without surgical trauma, and with preserved organ function. Of notice, the outcome for these patients was better than for cardiogenic shock in general Citation[17]. The use of the Impella in these patients provides ventricular unloading in combination with increased coronary flow, which is beneficial against hypoperfusion, both systemically and in the heart itself.
The majority of the non-surviving patients in the surgical group died within 30 days of implantation. The survivors had a higher CO 12 hours postoperatively compared to the non-survivors, and lower filling pressures and higher SvO2 among survivors probably indicate the importance of an adequate end-organ circulation. This is in concordance with Siegenthaler et al. Citation[9], who described a residual cardiac function (CO minus pump-CO) of 1 L/min or more measured 2 hours postoperatively to be a predictor of survival. Therefore, CO at 12 hours could be used as an early sign when planning further treatment for the patient, i. e. need for long-term assist or further surgery. Somewhat similar was the finding of Mendoza Citation[13] that the Cardiac Power Output (CPO) correlates with survival. The CPO is the product of mean arterial blood pressure and blood flow and this may provide a more practical indication of the actual delivery to the end-organ perfusion. The use of preoperative IABP favors survival in the surgical group of patients. Hemodynamic assessment using CPO and preoperative IABP confirm that the strategy of early intervention and optimized hemodynamics in the early postoperative period is favorable for long-term survival. This is confirmed in our study even though calculations were made using postoperative measurements. A higher SvO2 at 12 hours among the survivors at one year also indicates the benefit of adequately obtained peripheral perfusion. Early low SvO2 and CPO should stress the decision for further treatment options. This concept aids long-term prognosis in the early postoperative period.
The definition of right ventricular failure is difficult as reported by others Citation18–20. The diagnosis of RV-failure when weaning from CPB is complex when based on various hemodynamic and laboratory parameters, but experience indicate that the clinical observation is more obvious. Furthermore, RV-failure is associated with a high mortality Citation[20], both short- and long-term. In our series only eight patients were judged to need a RV-assist system, although several more were deemed to have suffered from right ventricular failure. The early recognition of this condition is important. When there is an indication for RVAD, the Impella RD may be inadequate due to limited flow capacity. Six of eight patients with the Impella RD in this series did not survive 30 days postoperatively and the long-term survival was also poor. The extensive use of echocardiography in the operating room and in the ICU has made it easier and more convenient to diagnose and follow the effect of treatment in right ventricular failure Citation[21]. In general, echocardiography plays a dominant role in the ongoing evaluation of ventricular function, flow pattern and pump positioning during the treatment with the device. The exact measurements of dimensions, ejection fractions and various indexes play a secondary role to changes over time combined with the observed hemodynamic condition. Early experience with the right ventricular Impella (RD) showed significant problems with plasma leakage from the device graft, but this inadequacy was corrected by the manufacturer.
Heart failure in combination with cardiac surgery affects coagulation. This is reflected in the surgical group where 50% of the patients were reoperated because of bleeding. This is higher than expected and unlike the results of other Impella centers even though others have reported high reoperation figures due to bleeding with other types of device Citation[18]. The degree of postoperative anticoagulation has been reduced in our centers to only 5.000 IU of heparin in the purge system. When postoperative bleeding cease, dalteparin sodium as anti-thrombotic prophylaxis is routine. The recorded infections in this series were mostly pneumonia, septicemia and wound infection. Device-related infections were not a problem, probably because of the short time they were in use. The groin approach with the LP 5.0 and LP 2.5 is advantageous in the ICU or in the catheter-lab setting since access is easy. However, it must be borne in mind that use over many days may compromise perfusion of the leg. In the early days of the Hemopump there were reports of difficulties with the groin approach due to arteriosclerosis Citation[5]. This was not, however, the experience in this series of Impella patients. In the OR setting, when inserting the LD-device, it is possible to close the sternum and skin to avoid colonization of the wound. The obvious drawback with this approach, however, is the need to reopen the sternum when removing the device.
The overall mortality in this group of patient was high. However, other reports Citation[9], Citation[12], Citation[14] have observed in-hospital mortality between 50% and 54% in postcardiotomy patients. The 30-day mortality for the postcardiotomy group in this report was 45%. The one-year mortality, however, was 64%, which illustrates the severity of heart failure in this patient group. Acceptable? No, there is much more to learn about the pathophysiology of acute heart failure and understand its treatment and timing to lower the mortality rates in the long term. The Impella Recover 2.5 has a great potential in high-risk PCI procedures due to its effectiveness and easy use.
Limitations
A retrospective analysis has obvious drawbacks. One of which is that important hemodynamics and laboratory data are not recorded due to the emergency nature of the situation and are therefore missing for some patients. A prospective study to gather robust data is required if one is to make conclusions regarding this novel treatment. Different Impella pumps were used in different clinical settings and the groups are thus not comparable.
Conclusion
Fifty patients were treated with the novel short-term axial flow pump Impella Recover® at three different cardiothoracic centers in Sweden. The one-year mortality in the surgical group was 64% and in the non-surgical group 29%. The majority of deaths occurred within 30 days of implantation. The survivors in the surgical group had a significantly higher cardiac output at 12 hours, higher cardiac power output at 6 and 12 hours and higher SvO2 at 12 hours postoperatively than the non-survivors. In patients with acute cardiogenic shock, regardless of cause, early intervention with short-term devices providing optimized hemodynamics is of importance for long-term survival. Insufficient early response to treatment urges the consideration of further available options. The percutaneous approach was very useful in the emergency situation. The stabilized patient could be considered for further evaluation regarding bridge-to-recovery, bridge-to-bridge or bridge-to-decision.
Disclosures
There are no disclosures
References
- Goldstein J, Oz M, Rose E. Implantable left ventricular assist devices. N Engl J Med. 1998; 339: 1522–33
- Frazier OH, Delgado RM. Mechanical circulatory support for advanced heart failure: Where does it stand in 2003?. Circulation 2003; 108: 3064–8
- Wampler RK, Moise JC, Frazier OH, Olsen DB. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 1988; 34: 450–4
- Frazier OH, Wampler RK, Duncan JM, Dear WE, Macris MP, Parvics SM, et al. First human use of the Hemopump, a catheter-mounted ventricular assist device. Ann Thorac Surg. 1990; 49: 299–304
- Lönn U, Peterzén B, Granfeldt H, Babic A, Casimir-Ahn H. Hemopump treatment in patients with postcardiotomy heart failure. Ann Thorac Surg. 1995; 60: 1067–71
- Apel J, Paul R, Klaus S, Siess T, Reul H. Assessment of hemolysis related quantities in a microaxial blood pump bycomputational fluid dynamics. Artif Organs. 2001; 25: 341–7
- Martin J, Benk C, Yerebakan C, Derjung G, Sarai K, Beyersdorf F. The new “Impella” intracardiac microaxial pump for treatment of right heart failure after orthotopic heart transplantation. Transplant Proc. 2001; 33: 3549–50
- Meyns B, Dens J, Sergeant P, Herijgers P, Daenen W, Flameng W. Initial experiences with the Impella device in patients with cardiogenic shock – Impella support for cardiogenic shock. Thorac Cardiovasc Surg. 2003; 51: 312–7
- Siegenthaler MP, Brehm K, Strecker T, Henke T, Notzold A, Olsekewski M, et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: A three-center experience. J Thorac Cardiovasc Surg. 2004; 127: 812–22
- Garatti A, Colombo T, Russo C, Lanfranconi M, Milazzo F, Catena F, et al. Different applications for left ventricular mechanical support with the Impella Recover 100 microaxial blood pump. J Heart Lung Transplant. 2005; 24: 481–5
- Valgimigli M, Steendijk P, Sianos G, Onderwater E, Serruys PW. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2.5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005; 65: 263–7
- Garatti A, Colombo T, Russo C, Lanfranconi M, Milazzo F, Catena F, et al. Left ventricular mechanical support with the Impella Recover left direct microaxial blood pump. Artif Organs. 2006; 30: 523–8
- Mendoza DD, Cooper HA, Panza JA. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J. 2007; 153: 366–70
- Ramnarine IR, Grayson AD, Dihmis WC, Mediratta NK, Fabri BM, Chalmers JA. Timing of intra-aortic balloon support and 1-year survival. Eur J Cardiothor Surg. 2005; 27: 887–92
- Reesink KD, Dekker AL, Van Ommen V, Solmers C, Geshes GC, van der Veen FH, et al. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest. 2004; 126: 896–902
- Baldwin RT, Radovancevic B, Conger JL, Matsuwaka R, Duncan JH, Vaugh WK, et al. Peripheral organ perfusion augmentation during left ventricular failure. A controlled bovine comparison between the Intraaortic Balloon Pump and the Hemopump. Texas Heart Inst J. 1993; 20: 275–80
- Mann HJ, Nolan PE, Jr. Update on the management of cardiogenic shock. Curr Opin Crit Care. 2006; 12: 431–6
- Miller CA, Pae WE, Pierce WS. Combined registry for the clinical use of mechanical ventricular assist devices: postcardiotomy cardiogenic shock. ASAIO Trans. 1990; 36: 43–6
- Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Fung J, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation 2002; 106(Suppl I)I-198–I-202
- Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery H, Oz M, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: A continuing problem. Ann Thorac Surg. 2002; 73: 745–50
- Catena E, Milazzo F, Merli M, Paino R, Garatti A, Colombo T, et al. Echocardiographic evaluation of patients receiving a new left ventricular assist device: The Impella recover 100. Eur J Echocardiography. 2004; 5: 430–7