Abstract
The comment concerns two short-term assist systems, namely the Impella axial-flow recovery system and extra-corporeal membrane oxygenation (ECMO) used for circulatory assistance in patients with acute heart failure. The results, particularly for patients in cardiogenic shock not related to cardiac surgery, calls for cautious optimism.
The recently published “Triumph” trial aimed to improve systemic medical treatment in cardiogenic shock through unselective NO-blockade using the compound tilarginine (ArgiNOx Pharmaceuticals) Citation[1]. The drug failed to improve survival, and the study was terminated early due to “no prospect of a positive effect”. In these patients with cardiogenic shock following myocardial infarction and subsequent revascularization, the short-term mortality was 45%. Ventricular assist devices (VAD) were used in 12% of the patients.
The “Triumph” trial benchmarks the present treatment status for cardiogenic shock following myocardial infarction; the drug based support treatment is inadequate and a high percentage of these gravely ill patients die in the acute phase of the shock. For these patients, the remaining treatment options are mechanical assist systems and/or heart transplantation. This is an area of cardiology and cardiac surgery where few controlled studies have been done, and where the future probably will not see a great many traditionally designed randomized trials. However, adding assist systems on top of revascularization, valve procedures, intra aortic balloon pumps (IABP) and vasoactive drugs, is not a “penicillin-like slam-bang effect” Citation[2], and outcome for patients treated with mechanical assist systems are far from optimal.
In this issue of the Journal, two reports from Sweden present recent results from the use of ECMO (extracorporeal membrane oxygenation) and the Impella® pump in acute heart failure Citation[3], Citation[4]. These two reports give important insight to the current status of short-term circulatory assist devices in Scandinavia. Importantly, they also provide a framework to address the many unsolved questions regarding indication, timing, patient selection, device selection, support treatment and treatment goals in both the short and long-term for mechanical assist devices.
ECMO (Extracorporeal Membrane Oxygenation)
Using an extracorporeal pump and membrane oxygenator (ECMO), i.e. a refined heart-lung machine, has a 30 year long history as a bail out treatment after cardiac surgery complicated by acute cardiac pump failure Citation[5]. This technology has gained a relatively established indication in the treatment of neonatal and pediatric lung failure Citation[6]. ECMO also represents an alternative for pediatric patients with post-surgical cardiac failure Citation[7], and pediatric cardiac failure of other origins Citation[8]. However, as a treatment for pulmonary failure in adults, the usefulness of this system has been more doubtful Citation[9], Citation[10]. The recently finished CESAR study randomized adults patients with lung failure to ECMO treatment at one specialized centre or “best treatment” at other intensive care institutions. Preliminary results from the study indicate a benefit for ECMO treatment (www.medscape.com/viewarticle/569740).
As a treatment for adult patients with acute cardiac pump failure, the experience has been mixed. Overall, the survival for patients in the largest patient series has been around 30% (www.elso.med.umich.edu/Registry) Citation[11], Citation[12]. Similar outcomes are reproduced in patient series with different indications ranging from cardiac arrest to patients with failure to wean from CPB (Cardiopulmonary bypass) Citation13–16. ECMO has also been used successfully as a bridge-to-bridge device for other VADs or cardiac transplant Citation[17]. Importantly, ECMO can serve as a universal tool in patients with right or biventricular heart failure Citation[18]. However, the conduct of this treatment remains a technical challenge and carries a substantial complication rate Citation[15].
In this context, the results presented by Linden and colleagues from the joint Salgrenska/Karolinska database, are encouraging Citation[3]. Particularly, the 63% survival among the 19 patients with non-operated cardiogenic shock hopefully represents a move forward. However, as the patient group is small (the 95% CI for survival is 41 – 85%), and the level of hemodynamic collapse is not stated, the results need to be evaluated cautiously. Compared to other series the time on ECMO for these patients (survivors 9.9 days) was relatively long and needs to be considered in particular for subsequent patients on ECMO.
Impella® axial flow pump
Hans Granfeldt and colleagues report on the use of the Impella assist system in 50 patients from three cardiothoracic centres in Sweden Citation[4]. In a majority of these patients the treatment goal was native heart recovery. The reported short term survival was again particularly promising in patients with cardiogenic shock without any prior surgery (79% 30 day survival, 95% CI 57 – 100%), also compared to patients with postcardiotomy heart failure (55%).
The effect of the Impella pump in terms of hemodynamic effectiveness and outcome is not clear. In the study presented, hemodynamic status prior to pump implantation was not systematically assessed and no strict criteria were used before therapy was applied. Unfortunately, the relation between the hemodynamic response to the pump and outcome could not be determined. Patients with the highest cardiac output and SVO2 after pump placement had the best long-term outcome, but it is unknown if this was a result of the device or just reflecting a better prepump cardiac function in these patients.
Without any strict hemodynamic criteria for applying the Impella or any control group receiving optimal conventional treatment or alternative ventricular assist systems, it is difficult to assess if optimal outcome or in fact any survival benefit was achieved with this system. The difficulty in assessing survival benefit is further illustrated from two randomized trials comparing the TandemHeart® percutaneous left ventricular assist device and IABP in the setting of cardiogenic shock Citation[19], Citation[20]. The assist device proved more efficient in improving hemodynamics compared to IABP, but this did not translate into improved survival for the LVAD patients. However, both of these studies were too small to allow an assessment of a possibly small to moderate effect on survival. Importantly, however, the incidence of complications was higher in the assist treated group Citation[19]. With these reservations, a 79% 30 day survival in cardiogenic shock is certainly promising compared to most patient series treated without assist devices.
Previous reports on the Impella pump used in cardiogenic shock with or without recent heart surgery, indicates that it is feasible and safe to use and has the potential to improve cardiac output, reduce ventricular filling pressures and thus reverse the shock Citation21–23. A small hemodynamic benefit could also be demonstrated in the randomized ISAR-SHOCK Trial comparing the Impella pump (LP2.5) with IABP Citation[24]. However, also in this study outcomes were unsatisfactory with a mortality of 45% and no mortality difference from the IABP group. In a report by Siegenthaler and collegues on postcardiotomy heart failure patients comparing the outcomes in Impella treated patients with IABP treated patients, the Impella pump seemed to improve outcome. However, they found clear indications for a limited effect of the Impella 5.0 pump when ventricular function was severely impaired Citation[25].
Detailed animal studies have been performed with the Impella pump's predecessor, the Hemopump®. The main conclusions from these studies were the pump's ability to lower myocardial VO2 in ischemic hearts through a reduction in the cardiac work load and sustained coronary perfusion Citation26–29. However, the ability of the Impella pump to restore systemic circulation in profound cardiogenic shock is uncertain Citation29–31.
Patient selection
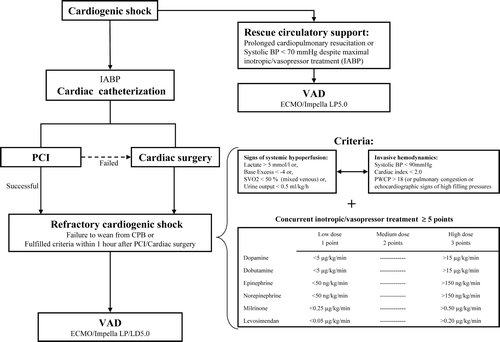
Cardiogenic shock and postcardiotomy cardiogenic shock include a spectre of patients with different prognosis and degrees of hemodynamic compromise. The nature of postcardiotomy cardiogenic shock with thorough monitoring and immediate treatment when symptoms of pump failure and shock develops has made it possible to develop tools to immediately grade the severity based on both inotropic demands and hemodynamic assessment Citation[32], Citation[33]. Such treatment algorithms can be beneficial and ensure that high risk individuals receive early intervention Citation[32]. The same approach should be helpful for selection of assist candidates with cardiogenic shock Citation[34]. Hemodynamic indices used in cardiogenic shock following AMI, such as stroke volume index (SVI), could also be useful in the selection of patients for assist devices Citation[35]. A selection of low risk patients for treatment with percutaneous short term assist devices such as the Impella and the use of more powerful assist devices in patients with more profound shock has been proposed Citation[36]. An example of a simple treatment algorithm is given in .
Where do we go from here?
The assumption that improved technological solutions and hemodynamics translates into improved survival may not be completely valid in these acutely ill patients. A cardiogenic shock could be partly caused by, and most certainly initiate a, “biological storm” in these patients Citation37–39. A number of active processes involving the myocardium, peripheral circulation and organ functions take place in the course of acute heart failure, and the assist devices themselves also affect these biological processes Citation[38], Citation[40]. In spite of the fact that complement activation was found to be initiated by CPB more than 25 years ago Citation[41], there are no convincing predesigned analyses indicating a benefit of blocking complement or other important mediators of systemic inflammation, like TNF Citation42–46. It is not too speculative to imagine, however, that additional knowledge of the pathophysiology of cardiogenic shock and post-pump failure must be obtained before we can pave ground for an improved medical treatment that ensures optimized organ perfusion in the short-term along with optimized recovery of both the heart and the patient. While technical improvements can bring us somewhat further, a more complete understanding of the biology involved is probably critical to further improvement of the outcomes for these patients.
The future use of short term circulatory assistance in Norway, and probably in the whole of Scandinavia, would benefit from a further development of a united and organized national assist program. This is a particular challenge for the clinics without a transplant or long term assist program, and these centres treat approximately 80% of the Norwegian adult cardiac surgical population. Data from the Norwegian registry for cardiac surgery (see ) reveals that the total number of acute assist systems used in 2006, was around one fifth of the conservatively estimated number needed for acute heart failure alone Citation[47]. It is also remarkable that Abiomed BVS 5000®, approved by the FDA and probably the most extensively used system world-wide for short-term ventricular assistance, is barely used in Scandinavia (three devices sold in Sweden, communicated from Abiomed). Non-transplant clinics need to establish an easy rescue program based on a robust pump system that can adequately provide the complete cardiac output with the option to integrate an oxygenator when needed to secure a biventricular assist function. A reasonably smooth and reliable transfer algorithm then needs to be established for transfer of both patients and pumps in case scenarios where this is needed.
Table I. Data from the Norwegian registry for cardiac surgery
We do not think that there will be a great many randomized trials involving acute heart failure patients and the application of different assist devices. The clinical scenarios and multifaceted treatments applied in this patient group make such a study design difficult to handle and with limited applicability Citation[48]. Ideally, the different centers should establish a registry and treatment algorithm for all patients with acute heart failure in order to keep track of the potential candidates for such treatments and monitor their outcome. The two studies presented in this issue of the “Journal” illustrates that improvements can be done in gathering clinical data concerning single patients. The best option to get useful clinical data in this group of patients would probably be a common “patient report form” with prespecified variables. As these patients demand extensive resources, the additional effort to obtain such data is scant. A national or Scandinavian coordinated effort to collect such data would certainly be a step forward.
Acknowledgements
We thank Professor Jan L. Svennevig, Rikshospitalet, Oslo for providing the data on assist use from the Norwegian registry of heart surgery. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, van der Werf F, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: The TRIUMPH randomized controlled trial. JAMA. 2007; 297: 1657–66
- Moses LE. The series of consecutive cases as a device for assessing outcome of interventions. Medical uses of statistics2nd ed, JC III Bailar, F Mosteller. NEJM Books, Boston, MA 2000; 125–141
- Linden, H, Wiklund, L, Haraldsson, Å, Berglin, E, Hultman, J, Dellgran, G. Temporary circulatory support with ECMO in adults with refractory cardiogenic shock. Scand Cardiovasc J. 2009, (this issue).
- Granfeldt, H, Hellgren, L, Dellgren, G, Myrdal, G, Wassberg, G, Kjellman, U, et al. The Impella® recovery axial-flow system for acute heart failure at three cardiothoracic centers in Sweden. Scand Cardiovasc J. 2009, (this issue).
- Bartlett RH, Gazzaniga AB, Fong SW, Jefferies MR, Roohk HV, Haiduc N. Extracorporeal membrane oxygenator support for cardiopulmonary failure. Experience in 28 cases. J Thorac Cardiovasc Surg. 1977; 73: 375–86
- UK Collaborative ECMO Trail Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996; 348: 75–82
- Chaturvedi RR, Macrae D, Brown KL, Schindler M, Smith EC, Davis KB, et al. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart. 2004; 90: 545–51
- Thourani VH, Kirshbom PM, Kanter KR, Simsic J, Kogon BE, Wagoner BE, et al. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) in pediatric cardiac support. Ann Thorac Surg. 2006; 82: 138–44
- Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979; 242: 2193–6
- Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994; 149: 295–305
- Nichol G, Karmy-Jones R, Salerno C, Cantore L, Becker L. Systematic review of percutaneous cardiopulmonary bypass for cardiac arrest or cardiogenic shock states. Resuscitation. 2006; 70: 381–94
- Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: Survival at five years. J Thorac Cardiovasc Surg. 2001; 122: 92–102
- Chen JS, Ko WJ, Yu HY, Lai LP, Huang SC, Chi NH, et al. Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med. 2006; 34: 950–7
- Doll N, Fabricius A, Borger MA, Bucerices J, Kramer K, Doll S, et al. Temporary extracorporeal membrane oxygenation in patients with refractory postoperative cardiogenic shock–a single center experience. J Card Surg. 2003; 18: 512–8
- Doll N, Kiaii B, Borger M, Bucerices J, Kramer K, Schmitt DV, et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg. 2004; 77: 151–7
- Chen YS, Lin JW, Yu HY, Ko WJ, Jeng JS, Chang WT, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet. 2008; 372: 554–61
- Hoefer D, Ruttmann E, Poelzl G, Kilo J, Hoermann C, Margreiter R, et al. Outcome evaluation of the bridge-to-bridge concept in patients with cardiogenic shock. Ann Thorac Surg. 2006; 82: 28–33
- Taghavi S, Zuckermann A, Ankersmit J, Wieselhaker G, Rajek A, Laufer G, et al. Extracorporeal membrane oxygenation is superior to right ventricular assist device for acute right ventricular failure after heart transplantation. Ann Thorac Surg. 2004; 78: 1644–9
- Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005; 26: 1276–83
- Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006; 152: 469–8
- Meyns B, Dens J, Sergeant P, Herijgers P, Daenen W, Flameng W. Initial experiences with the Impella device in patients with cardiogenic shock – Impella support for cardiogenic shock. Thorac Cardiovasc Surg. 2003; 51: 312–7
- Jurmann MJ, Siniawski H, Erb M, Drews T, Hetzer R. Initial experience with miniature axial flow ventricular assist devices for postcardiotomy heart failure. Ann Thorac Surg. 2004; 77: 1642–7
- Garatti A, Colombo T, Russo C, Lanfranconi M, Milazzo F, Catena E, et al. Left ventricular mechanical support with the Impella Recover left direct microaxial blood pump: A single-center experience. Artif Organs. 2006; 30: 523–8
- Seyfarth M, Sibbing D, Bauer I, Frolich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008; 52: 1584–8
- Siegenthaler MP, Brehm K, Strecker T, Hanke T, Notzold A, Olschewski M, et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: A three-center experience. J Thorac Cardiovasc Surg. 2004; 127: 812–22
- Merhige ME, Smalling RW, Cassidy D, Barrett R, Wise G, Short J, et al. Effect of the hemopump left ventricular assist device on regional myocardial perfusion and function. Reduction of ischemia during coronary occlusion. Circulation. 1989; 80: 158–66
- Baldwin RT, Radovancevic B, Conger JL, Matsunaka R, Duncan JM, Vaughn WK, et al. Peripheral organ perfusion augmentation during left ventricular failure. A controlled bovine comparison between the intraaortic balloon pump and the Hemopump. Tex Heart Inst J. 1993; 20: 275–80
- Smalling RW, Cassidy DB, Barrett R, Lachterman B, Felli P, Amirian J. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. A comparison with intra-aortic balloon counterpulsation and reperfusion alone in a canine infarction model. Circulation. 1992; 85: 1152–9
- Scholz KH, Hering JP, Schroder T, Uhlig P, Kreuzer H, Hellige G. Left-ventricular unloading by transvalvular axial flow pumping in experimental cardiogenic shock and during regional myocardial ischemia. Cardiology. 1994; 84: 202–10
- Sauren LD, Accord RE, Hamzeh K, de Jong M, van der Nagel T, van der Veen FH, et al. Combined Impella and intra-aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: An experimental study. Artif Organs. 2007; 31: 839–42
- Marks JD, Pantalos GM, Long JW, Kinoshita M, Everett SD, Olsen DB. Myocardial mechanics, energetics, and hemodynamics during intraaortic balloon and transvalvular axial flow hemopump support with a bovine model of ischemic cardiac dysfunction. ASAIO J. 1999; 45: 602–9
- Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: Experience with the Abiomed BVS system. J Card Surg. 1999; 14: 288–93
- Hausmann H, Potapov EV, Koster A, Krabatsch T, Stein J, Yeter R, et al. Prognosis after the implantation of an intra-aortic balloon pump in cardiac surgery calculated with a new score. Circulation. 2002; 106: 203–6
- Samuels LE, Holmes EC, Thomas MP, Entwistle JC, Morris RJ, Narula J, et al. Management of acute cardiac failure with mechanical assist: Experience with the ABIOMED BVS 5000. Ann Thorac Surg. 2001; 71: 67–72
- Jeger RV, Lowe AM, Buller CE, Pfisterer ME, Dzavik V, Webb JG, et al. Hemodynamic parameters are prognostically important in cardiogenic shock but similar following early revascularization or initial medical stabilization: A report from the SHOCK trial. Chest. 2007; 132: 1794–803
- Potapov EV, Loforte A, Weng Y, Jurmann M, Pasic M, Drew ST, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008; 3: 185–94
- Felker GM, Cotter G. Unraveling the pathophysiology of acute heart failure: An inflammatory proposal. Am Heart J. 2006; 151: 765–7
- Hochman JS. Cardiogenic shock complicating acute myocardial infarction: Expanding the paradigm. Circulation. 2003; 107: 2998–3002
- Kohsaka S, Menon V, Lowe AM, Lange M, Dzavik V, Sleeper LA, et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. 2005; 165: 1643–50
- Graulich J, Sonntag J, Marcinkowski M, Bauer K, Kossel H, Buhrer C, et al. Complement activation by in vivo neonatal and in vitro extracorporeal membrane oxygenation. Mediators Inflamm. 2002; 11: 69–73
- Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983; 86: 845–57
- Eikelboom JW, O'Donnell M. Pexelizumab does not “complement” percutaneous coronary intervention in patients with ST-elevation myocardial infarction. JAMA. 2007; 297: 91–2
- Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, et al. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008; 136: 884–93
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003; 107: 3133–40
- Haverich A, Shernan SK, Levy JH, Chen JC, Carrier M, Taylor KM, et al. Pexelizumab reduces death and myocardial infarction in higher risk cardiac surgical patients. Ann Thorac Surg. 2006; 82: 486–92
- Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004; 109: 1594–602
- Hermansen SE, Hansen M, Roaldsen M, Muller S, How OJ, Myrmel T. How many acute heart failure patients need a ventricular assist device?. Scand Cardiovasc J. 2008; 42: 118–24
- Bachet J. The potatoes and the bottle. Eur J Cardiothorac Surg. 2004; 25: 242–245