Abstract
Objectives. Acute STEMI is routinely treated by acute PCI. This treatment may itself damage the tissue (reperfusion injury). Conditioning with GLP-1 analogs has been shown to reduce reperfusion injury. Likewise, ischemic postconditioning provides cardioprotection following STEMI. We tested if combined conditioning with the GLP-1 analog liraglutide and ischemic postconditioning offered additive cardioprotective effect after reperfusion of 45 min coronary occlusion of left anterior descending artery (LAD). Design. Fifty-eight non-diabetic female Danish Landrace pigs (60 ± 10kg) were randomly assigned to four groups. Myocardial infarction (MI) was induced by occluding the LAD for 45 min. Group 1 (n = 14) was treated with i.v. liraglutide after 15 min of ischemia. Group 2 (n = 17) received liraglutide treatment concomitant with ischemic postconditioning, after 45 min of ischemia. Group 3 (n = 15) recieved ischemic postconditioning and group 4 (n = 12) was kept as controls. Results. No intergroup differences in relative infarct size were detected (overall mean 57 ± 3%; p = 0.68). Overall mortality was 34% (CI 25–41%) including 26% post-intervention, with no intergroup differences (p = 0.99). Occurrence of ventricular fibrillation (VF) was 59% (CI 25–80%) including 39% postintervention with no intergroup differences (p = 0.65). Conclusions. In our closed-chest pig-model, we were unable to detect any cardioprotective effect of liraglutide or ischemic postconditioning either alone or combined.
Introduction
Primary percutaneous coronary intervention (pPCI) is widely used to treat acute ST-segment elevation myocardial infarction (STEMI) in order to establish vital reperfusion. Following revascularization, however, reperfusion may in itself cause myocardial damage,[Citation1,Citation2] and reperfusion injury may contribute to as much as 50% of the final infarct size.[Citation2] Several treatment methods have been proposed to prepare the myocardium for reperfusion injury.
Pharmacological conditioning includes preconditioning, in which the drug is given prior to the infarction, and examines the effect in patients already treated with the drug of interest,[Citation3–6] and postconditioning, in which the drug is administered prior to reperfusion and examines the effect in an acute setting.[Citation6–12] Native GLP-1 and GLP-1 analogs have been proposed to possess cardioprotective properties most likely through preservation of myocardial mitochondria during ischemia and reperfusion,[Citation11,Citation13] Most studies have shown cardioprotective effect of treatment with native GLP-1 and GLP-1 analogs both in animal models,[Citation5,Citation6,Citation9,Citation10,Citation12] with liraglutide specifically,[Citation4] and in humans.[Citation8] Results are, however, not consistent.[Citation3,Citation7,Citation9,Citation11]
Ischemic postconditioning is a method at which the ischemic myocardium is subjected to brief periods of subsequent ischemia at onset of reperfusion to attenuate reperfusion injury. The method has demonstrated infarct-reducing effect in animal experiments [Citation1,Citation2,Citation14–16] and in humans.[Citation1,Citation2,Citation14, Citation17–19] Presently, there is no consensus on the postconditioning protocol used, but three to four cycles of reperfusion and ischemia lasting 30–60 s are reportedly cardioprotective.[Citation14–19]
In a closed-chest porcine model, we tested the effect of pharmacological postconditioning with the GLP-1 analog liragutide administered shortly after experimental induction of an acute MI on myocardial salvage. We also tested whether an additive effect of concomitant treatment with ischemic postconditioning exists.
Methods
This randomized, placebo-controlled experiment was performed at the Department of Experimental Medicine at the University of Copenhagen.
Ethics
The animal procedures were performed conform the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes, and permission was granted by the Danish Animal Experiment Inspectorate, Ministry of Justice (date of approval: 2 November 2012, J-nr. 2012-15-2934-00288).
Anesthesia
The pigs were anesthetized during the entire experiment. Anesthesia was induced by an intramuscular injection of 1 ml/10 kg mixture of Zoletil®, which contains a 5 ml mixture of 125 mg tiletamine and 125 mg zolazepam with the addition of 6.5 ml Narcoxyl® (xylazine 20 mg/ml), 1.5ml Ketaminol® (ketamine 100 mg/ml), 2 ml Torbugesic and 2 ml methadone. Anesthesia was sustained with propofol (1.5 ml/kg/h). Prior to the PCI procedure and prior to extraction of the hearts, the pigs were given an intravenous bolus of 5 ml fentanyl for analgesia. Prior to the PCI procedure all pigs were given an intravenous bolus of 5 ml fentanyl for analgesia and an intravenous bolus injection of heparine (5000 IE) and amiodarone (Cordarone®, Sanofi-Aventis, 150 mg) to prevent thrombosis formation and arrhythmias.
Experimental protocol
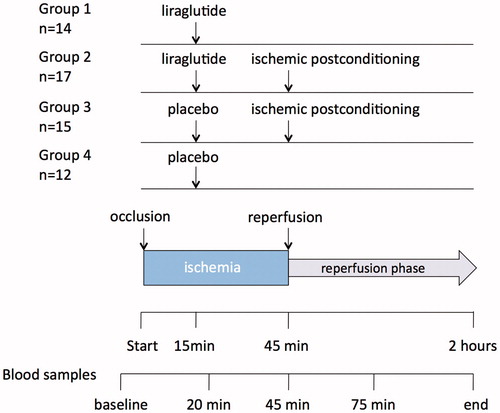
A total of 58 nondiabetic female Danish Landrace pigs (60 ± 10kg, aged 16–17 weeks) were included. The pigs were randomized into four blocks according to the four different treatments (). Group 1 (n = 14) was assigned to liraglutide only, and was treated with an intravenous infusion of liraglutide (Victoza®, Novo Nordisk, Denmark) 4.8 mg/ml with a flow rate of 6 ml/min after 15 min of ischemia until the onset of reperfusion, i.e. 30 min. Group 2 (n = 17) was assigned to liraglutide and ischemic postconditioning, and was treated with infusion of liraglutide as described as well as ischemic postconditioning at the onset of reperfusion. Group 3 (n = 15) was assigned to receive ischemic postconditioning treatment only, and group 4 (n = 12) was assigned to placebo treatment with intravenous saline (NaCl isotonic fluid, 9 mg/ml) equivalent to the amount of fluid infused in two liraglutide groups and the groups with only postconditioning.
Figure 1. Study design with four groups assigned to different treatments, as well as timing of blood sampling during the experiment.
Ischemia
According to the closed-chest porcine model MI was induced via PCI technique as previously described.[Citation20] The left anterior descending artery (LAD) was occluded just distal to the first diagonal, corresponding to a mid-LAD occlusion, for 45 min followed by deflation of the balloon. The period thus represented the time of ischemia.
Pharmacological postconditioning
Pharmacological postconditioning was performed by the infusion of 4.8 mg/ml liraglutide at a flow rate of 6ml/min after 15 min of ischemia until reperfusion. Liraglutide is equipotent to native GLP-1. With an anticipated volume of distribution of 4500–5000 ml and a protein binding of 99% this algorithm was chosen to obtain a plasma concentration of 10 nM liraglutide. Due to a high amount of protein binding (approximately 99%) the free liraglutide plasma concentration was not expected to decline more than 20% within the following 75 min of postreperfusion, therefore the plasma concentration was assumed to remain sufficiently constant throughout the experiment. This holds particularly true in the first few minutes of reperfusion during which the reperfusion injury is believed to take place.[Citation1,Citation2]
Ischemic postconditioning
In the pigs assigned to ischemic postconditioning, the balloon was deflated after 45 min of occlusion, and the artery kept open for 30 s until reinflation of the same balloon. Inflation/deflation was repeated in four cycles with intervals of 30 s.[Citation19]
Reperfusion
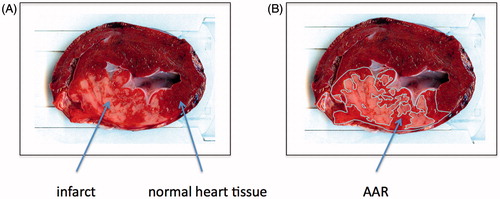
Following the ischemic period and the conditioning treatments, all groups were reperfused for 75 min. A sternum split of the thorax was then performed, the occlusion of LAD was located, and LAD was ligated just proximal to the occlusion with 3-0 Vicryl. The myocardium was dyed in vivo via injection of 50 ml, 2% Evans Blue (Sigma-Aldrich, St. Louis, MO) into the left ventricle ().
Figure 2. (A) Representative heart slice after staining with Evans Blue (Sigma-Aldrich) and 2,3,5-triphenyltetrazodiumchloride TTC (Sigma-Aldrich). Yellow-white areas indicate infarct, bright red areas indicate AAR and purple areas indicate normal heart tissue (see arrows for explanation). (B) Heart slice after the areas have been drawn.
Tissue preparation
Hereafter all animals were euthanized by extraction of the heart. The heart was sliced into 10 mm slices parallel to the atrioventricular plane, until reaching the occlusion site, to ensure that all the area at risk (AAR) was included[Citation20] (). The slices were then photographed and immediately hereafter prepared in a 1 L, 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, St. Louis, MO) for 20 min. This enabled visualization of the AAR and the necrotic myocardial tissue. TTC dyes the metabolically active tissue, whereas in necrotic tissue the staining does not occur [Citation20] (). The slices were then scanned (HP Scanjet G3010 Photo Scanner) along with a ruler, for later to establish the sizes of the areas of interest, i.e. AAR and infarct.
Arrhythmias
During the experiment, the time and occurrence of ventricular fibrillation (VF) and subsequent treatment were noted. VF was treated with DC-conversion and intravenous bolus injections of amiodarone (Cordarone®, Sanofi-Aventis) (300 mg) and bolus injections of adrenaline (1 mg) repeated as needed.
Assessment of infarct size and AAR
The AAR and infarct sizes were manually measured using Adobe Photoshop CS6 by two individual observers blinded to the treatment groups. The areas were drawn by hand and the sizes calculated in mm2 by the program. A strong positive correlation between the two blinded observers, indicate that there was a mutual consensus on the areas measured. (r = 0.88, p < 0.0001). Afterwards a third blinded observer confirmed the measurements. The values obtained were used to express the infarct size as a percentage of the AAR (infarct/AAR ratio (%)) [Citation20,Citation21] ().
Five blood samples were taken during the experiment: at baseline, after 20 min of ischemia, at the end of ischemia, after 75 min and finally at the end of the experiment to measure troponin T (TnT) levels (). Two plasma samples, two serum samples and one full blood sample were taken at each point. The plasma and serum samples were centrifuged at 3000 rpm, the supernatant extracted and frozen initially with dry ice and subsequently stored at −80 °C for later analyses. TnT was analyzed using Sandwich Electrochemiluminescence-immunoassay (ECLIA) at Rigshospitalet, Denmark.
Sample size and statistical analyses
The sample size calculation was based on the assumption that the infarct/AAR-ratio in the control group (group 4) would be 50% and that active treatment with either liraglutide (group 1), liraglutide and ischemic postconditioning (group 2), and ischemic postconditioning alone (group 3) would reduce the infarct size by 20% with a standard deviation of 7.5% (L. Timmers, personal communication). With a power of 80% and a 5% risk of type 1 error we needed nine pigs in each group in order to detect a statistically significant intergroup difference. These estimations were based on a similar work employing the same model.[Citation10]
The study had a 2 × 2 factorial design. All analyses were performed with IBM® SPSS® Statistics, Version 20.0 (IBM Corp., Armonk, NY). The AAR, infarct sizes as well as the TnT values are normally distributed and are analyzed with one-way ANOVA to establish intergroup variance. The infarct/AAR ratios are not normally distributed, and thus data was analyzed by the Kruskal–Wallis test for more than two independent samples. Mann–Whitney tests were used to evaluate any intergroup differences between individual means, while infarct/AAR-ratios and TnT values were analyzed by Student’s t-test. Intergroup differences in relation to mortality and occurrence of VF were analyzed using Fisher’s exact test. A p-value <0.05 (2-sided) was considered statistically significant. Values are presented as mean ±1 SEM.
Results
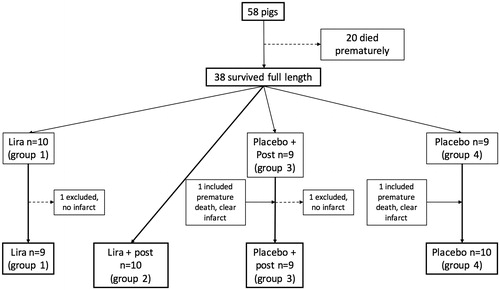
A total of 38 out of the 58 pigs included in the experiment survived the full length of the experiment. Two pigs, one each from group 1 and 3, respectively, were excluded due to no infarct on scan. Two pigs, each one from group 3 and 4, respectively, died in the reperfusion phase (at the 73 and 104 min), but have been included in the analyses as there were clear infarcts on the scans (). The overall mortality was 34% (CI 25–41%); group 1 n = 4 (29%), group 2 n = 7 (41%), group 3 n= 6 (40%), group 4 n = 3 (25%). Five deaths occurred prior to intervention (before 15 min) as a direct consequence of the catheterization procedure or the ischemic injury itself and were therefore not attributed to any assigned treatment group. This yields an overall mortality of 26% after commencement of active treatment or placebo. There was no statistical significant difference in mortality between the groups (p = 0.99) with Fisher’s exact test.
Figure 3. Flowchart showing inclusions and exclusions during the trial, as well as the final number of pigs in each group. Two pigs, one each from group 1 and 3 respectively, were excluded due to no infarct on scan. Two pigs, one from group 3 and 4, respectively, died in the reperfusion phase (at the 73rd and 104th minute), but are included in the analyses as clear infarcts were seen on the scans.
Overall VF occurred in 59% (CI 25–80%) of the pigs. Eleven first occurrences of VF took place prior to intervention (before 15 min), again believed to be a direct consequence of the catheterization procedures and the ischemic injury and were therefore not attributed to any subsequent treatment with active treatment or placebo. Thus, after commencement of active treatment, VF occurred for the first time in 39% of the cases. There was no statistical significant difference in occurrence of VF between the groups (p = 0.65).
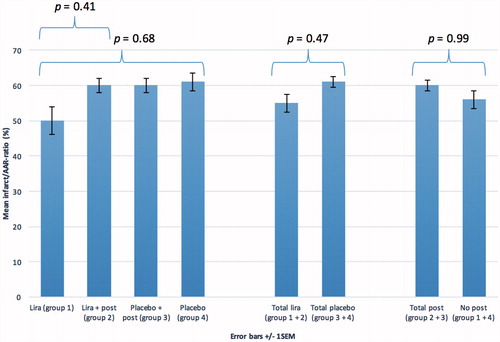
The AAR size was comparable in all four groups (overall mean 2246 ± 142 mm2; p = 0.66), indicating that all groups were subjected to the same extent of ischemia. The infarct sizes did not differ between groups (overall mean 1385 ± 128 mm2; p = 0.69). There were no intergroup differences in the infarct/AAR-ratios (mean 57 ± 3% (SD 17%); p = 0.68) (), indicating that there was no difference in the relative infarct sizes between the four groups. Increasing sample size by grouping the liraglutide pigs to analyze the effect of liraglutide treatment (groups 1 and 2) versus no liraglutide treatment (groups 3 and 4) did not result in a statistically significant effect of liraglutide compared to controls (p = 0.47) (). Similarly, an analysis of the effect of ischemic postconditioning treatment (groups 2 and 3) versus no treatment (groups 1 and 4) did not show an effect of postconditioning compared with the controls (p = 0.99) ().
Figure 4. Mean infarct/AAR ratios in the four different groups and p values for the intergroup variation as well as pooled data regarding total liraglutide compared to total placebo and total postconditioning compared to no postconditioning. Data are presented as ±1 SEM. Lira: liraglutide; Post: postconditioning.
TnT levels at the end of the experiment showed no intergroup differences (overall mean 6421 ± 978 ng/l; p = 0.93). TnT was positively correlated with infarct/AAR-ratio (r = 0.53, p = 0.001). Hemolyses occurred in two of the samples, one from group 2 and 3, respectively, and the samples could not be analyzed. When similar tests, as performed on the infarct/AAR ratios, are performed on the TnT values, data show that there are no differences between the four groups, either when merging the sample sizes with respect to liraglutide treatment (group 1 and 2) compared to placebo (group 3 and 4) (p = 0.19), or ischemic postconditioning (group 2 and 3) versus no treatment (group 1 and 4) (p = 0.13) ().
Table 1. TnT Concentrations at the end of the experiment after liraglutide treatment and/or ischemic postconditioning after ischemia-reperfusion injury in the closed-chest porcine model. Data are presented as mean ±1 SEM.
Discussion
Neither liraglutide nor ischemic postconditioning nor the combined treatment significantly reduced the relative infarct size compared to placebo in a closed-chest porcine model of acute MI. The overall mortality of 34% is comparable to similar studies employing the closed-chest porcine model with a mean mortality of 17% (11–33%) based on a review of eight different studies.[Citation22] Occurrence of VF in 59% of the cases in our study is also comparable to similar studies employing the closed-chest porcine model with a mean occurrence of VF of 39% (10–87%) based on eight studies.[Citation22]
Experiments using rodent [Citation4–6,Citation9,Citation12] and porcine models [Citation10] and a human study [Citation8] have shown that native GLP-1[Citation5,Citation6] and GLP-1 analogs [Citation9,Citation10,Citation12] reduce infarct size either as pretreatment [Citation4–6] or acutely.[Citation6,Citation9,Citation10,Citation12] Although there is a discrepancy between the results of this study and those described in the literature, the reports of treatment results with native GLP-1 and GLP-1 analogs are not consistent. Another porchine study failed to show an effect of recombinant GLP-1 (7–36) treatment in reducing infarct size [Citation7] and failure of GLP-1(9–36) to show infarct reducing effect was found in a rat model of ischemia-reperfusion injury.[Citation9] Subcutaneously administered liraglutide before ischemia also failed to reduce infarct size in pigs.[Citation3] Moreover, liraglutide infusion at the onset of reperfusion after 30 min of ischemia in an isolated perfused mouse heart did not reduce infarct size.[Citation11]
Some possible explanations of the discrepant findings exist. In the only other porchine study which also did not find an infarct-reducing effect of liraglutide,[Citation3] plasma values of liraglutide were very low prior to occlusion. This indicates that the plasma concentration of liraglutide at the time of the ischemic insult may play a pivotal role in the cardioprotective efficacy. A review of the cardiovascular effects of GLP-1 [Citation11] suggests that a cardioprotective effect of liraglutide requires the heart to function with its normal neural, humoral and vascular inputs, indicating that liraglutide may not be efficient under ischemic conditions. Furthermore, Kristensen et al. [Citation3] suggested that the porcine model might be less sensitive for demonstrating cardioprotection compared to the rodent model. We speculate that a possible cardioprotective effect of liraglutide is not as pronounced as that of exenatide, other GLP-1 analogs or native GLP-1. These all reduce relative infarct size between 30–58%,[Citation5,Citation6,Citation8–10,Citation12] as compared with our finding of a nonsignificant reduction in infarct size of 18% compared to placebo in the liraglutide-treated group (group 1). It is not likely that concomitant treatment with liraglutide eliminated the effect of ischemic postconditioning, since sole treatment with ischemic postconditioning also failed to show an effect on infarct size.
All together the conflicting findings may suggest that GLP-1 analogs do not offer a class effect with regard to cardioprotection. Alternatively drug concentration, time of ischemia and the animal model chosen could be very critical issues.
The failure of the present study to show an effect of ischemic postconditioning may involve several factors. Our postconditioning protocol with 4 cycles of 30 s reperfusion/occlusion was based on our human study,[Citation19] which showed a reduction in relative infarct size. Moreover, ischemic postconditioning has been proven efficient in human studies with protocols of 4 cycles and 60 s of reperfusion/occlusion.[Citation17,Citation18] Other reasons for the lack of effect must be sought.
Overall our results suggest a methodological explanation for the lack of effect since both liraglutide and ischemic postconditioning failed to protect the myocardium. The final injury of the myocardium following ischemia and reperfusion is a combination of both ischemia (necrosis) and reperfusion injury. If the ischemic insult is very large (proximal LAD occlusion and/or long time of ischemia), reperfusion injury may proportionally contribute little to the final myocardial damage. A proposed effect of conditioning against reperfusion injury may thus be blunted. The high occurrence of VF of 59% in our study suggests large ischemic insults. We had a mean infarct size in the control group (group 4) of 61%, possibly leaving little potential to salvage the myocardium due to a small contribution of reperfusion injury to the final infarct size. This leads to the natural conclusion that any efforts to treat reperfusion injury may have a limited window of opportunity. If the relative infarct size has reached too high a percentage, it seems to be futile to commence treatment.
A proximal occlusion site results in a large area at risk and therefore a comparable high risk of VF. In this view, a more distal site could have been advantageous to stabilize the model. In addition, a shorter time of ischemia would have reduced the extent of the ischemic insult. Thus, the share of the total injury taken up by the reperfusion injury would have been larger and subsequently any treatment effect on this component is easier to detect.
Area at risk between groups was not different indicating that the occluding technique was robust. However, to further secure this it could have been nice to have measured LV function before and after occlusion.
Our study has strengths. The closed-chest porcine model is very similar to the actual clinical setting for the treatment of acute MI,[Citation22] making the results easier to extrapolate to humans. Our results with regard to SD of the relative infarct size, mortality and occurrence of VF are comparable to other studies employing the same model. The study also has limitations. The SD of the relative infarct size proved to be 17% in our study and not 7.5% as estimated based on a similar work.[Citation10] Thus, we need a considerably larger sample size in order to either confirm or reject the hypothesis of a 20% reduction in relative infarct size with active treatment. A post-hoc calculation showed that we would need 46 pigs in each group to detect a possible 20% reduction, and that we have power to detect a 38% reduction in relative infarct size. The SD for the relative infarct size in our study is, however, similar to other recent works employing the same model [Citation3,Citation10,Citation15] with an SD of 10–20%, indicating that the data in our study are comparable to similar studies.
In conclusion, our data does neither support a cardioprotective effect of liraglutide nor of ischemic postconditioning nor of the combined treatment in the setting of acute MI in pigs using a closed-chest model. Since in several species and studies, although not invariably, both ischemic postconditioning and GLP-1 analogs have been shown to reduce myocardial damage, our data may suggest that duration of ischemia and very proximal LAD-occlusion and thus large infarct sizes are important factors that blunt the ability to protect against reperfusion injury. Furthermore, the concentration of liraglutide at a major ischemic insult, experimental setup and treatment regimen (pretreatment or acute treatment) may play an important role in the cardioprotective efficacy of the highly protein-bound liraglutide, which may not be as pronounced as that of exenatide, other GLP-1 analogs or native GLP-1. Further studies are required with larger sample sizes in order to make final conclusions on the cardioprotective properties of liraglutide and concomitant treatment with ischemic postconditioning. Moreover, studies examining the window of opportunity with regards to conditioning treatment of reperfusion injury as well as the optimal postconditioning protocol are warranted.
Funding information
The work was funded by research grants from Nordsjællands Hospital Hillerød and grants from Arvid Nielssons Foundation and the A.P. Møller Foundation.
Acknowledgements
Helga Schultz, Marie Moth Henriksen, Ditte Madsen Andersen, Christian Frandsen, Marie-Louise Allingbjerg, Jon Bjørn, Camilla Bang Jespersen, Annika Crone Boelsmand Søndergaard, Christoffer Lundsgaard, Nadja Rossau, Kirstine Roll Vestergaard, Jacob Hartmann and Anne Agersted are thanked for assistance during the experiments.
Disclosure statement
Ulrik Pedersen-Bjergaard: Novo Nordisk Advisory Board.
Thomas Engstrøm: Novo Nordisk Advisory Board and speaker.
References
- Sharma V, Bell RM, Yellon DM. Targeting reperfusion injury in acute myocardial infarction: a review of reperfusion injury pharmacotherapy. Expert Opin Pharmacother. 2012;13:1153–1175.
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135.
- Kristensen J, Mortensen UM, Schmidt M, et al. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc Disord. 2009;9:31.
- Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983.
- Bose AK, Mocanu MM, Carr RD, et al. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151.
- Bose AK, Mocanu MM, Carr RD, et al. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11.
- Kavianipour M, Ehlers MR, Malmberg K, et al. Glucagon-like peptide-1 (7-36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003;24:569–578.
- Lonborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2011;33:1491–1499.
- Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249.
- Timmers L, Henriques JP, de Kleijn DP, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510.
- Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215.
- Wohlfart P, Linz W, Hubschle T, et al. Cardioprotective effects of lixisenatide in rat myocardial ischemia-reperfusion injury studies. J Transl Med. 2013;11:84.
- Ravassa S, Zudaire A, Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94:316–323.
- Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the working group of cellular biology of the heart of the European society of cardiology. Cardiovasc Res. 2010;87:406–423.
- Skyschally A, Walter B, Schultz Hansen R, et al. The antiarrhythmic dipeptide ZP1609 (danegaptide) when given at reperfusion reduces myocardial infarct size in pigs. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:383–391.
- Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588.
- Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148.
- Thuny F, Lairez O, Roubille F, et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:2175–2181.
- Lonborg J, Kelbaek H, Vejlstrup N, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41.
- Suzuki Y, Lyons JK, Yeung AC, et al. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv. 2008;71:100–107.
- Diemar SS, Sejling AS, Iversen KK, et al. Influence of acute glycaemic level on measures of myocardial infarction in non-diabetic pigs. Scand Cardiovasc J. 2015;49:376–382.
- Busch SV, Halladin NL, Jensen SE, et al. Closed-chest porcine model is superior in intervention studies of ischaemia reperfusion injury. Ugeskr Laeger. 2012;174:1807–1810.
