Abstract
Objectives. The mechanisms of the location and extension of acute aortic dissection (AD) are only poorly understood. The aim of this study was to compare the cohesion of the non-coronary aortic sinus (NAS) and the ascending aortic wall (AA) using the Dissectometer – a new device for analyses of the mechanical properties of the aorta. Design. The properties of the aortic wall were analyzed with the “Dissectometer” (parameters P7, P8 and P9) in adult patients undergoing aortic root (AR) replacement in two different segments: NAS and AA. The aortic wall thickness (AWT) was measured with a micrometer. Results. Thirty-three adult patients (mean age 65 ± 14 years, 80% male) were included in this study. The aortic wall of the NAS was significantly thinner than that of the AA (1.9 ± 0.4 vs. 2.3 ± 0.4, p < 0.01). In contrast, mechanical stability assessed by cohesion testing was diminished in AA samples compared to NAS samples (P7: 86.0 ± 55.0 vs. 152.3 ± 89.2, p < 0.01; P8: 2.5 ± 1.3 vs. 6.0 ± 3.1, p < 0.01; P9: 3.6 ± 1.4 vs. 7.8 ± 3.2, p < 0.01). Conclusions. This study shows that the wall of the AR is characterized by a thin but stable wall, whereas AA was found to be weaker despite its greater thickness. This difference might be involved in the development and spreading of aortic dissections.
Introduction
Acute aortic dissection (AD) is a life-threatening disease with a reported mortality as high as 1% per hour over the first two days.[Citation1] The initiating event in aortic dissections is a tear in the intimal lining of the aorta that may extend proximally, distally or both, with possible malperfusion and consecutive ischemia of organs.[Citation2] Thus, the prognosis of patients with AD depends mainly on the location and extension of the dissection. The vast majority of aortic dissections originate with an intimal tear in either the ascending aorta (65%), the aortic arch (10%), or the descending thoracic aorta distal to the ligamentum arteriosum (20%).[Citation3] It is not well investigated though why the ascending aorta seems to be more vulnerable to dissections than other segments of the aorta. Furthermore, it is quite unknown why the spreading of the dissection into the aortic arch is more common than retrograde dissection into the aortic root (AR).
The aortic diameter, as measured by CT angiography, has been used clinically as the main indication to perform elective aortic replacement, in order to prevent future dissections or rupture. However, hemodynamic parameters (e.g. pressure and wall shear stress), geometrical factors and the composition of the aortic wall are known to substantially affect disease formation and progression.[Citation4] Whereas several studies investigated mechanical, histological and hemodynamic properties mainly comparing healthy and diseased aorta,[Citation5–7] the differences in wall quality between different regions of the thoracic aorta, e.g. aortic sinus and ascending aorta, and their role in genesis and spreading of aortic dissections have not been investigated in detail so far.[Citation8] We previously reported about the inverse relation of aortic wall thickness (AWT) and aortic wall strength in the ascending segment with a thicker wall being less resistant to dissection.[Citation9] Therefore, we hypothesized that aortic wall properties assessed by cohesion testing and wall thickness differ between the ascending part of the aorta and the AR.
Material and methods
Study design
The study was approved by the Institutional Review Board and patients’ written informed consent was obtained. This single-center, non-randomized study included 33 consecutive patients who underwent open heart surgery with AR replacement at the West-German Heart and Vascular Center Essen between March 2010 and February 2013.
Surgery and sample collection
Surgery was carried out through a median sternotomy with cannulation of the ascending aorta. The AR was excised and two samples of the aortic wall were harvested using a rounded knife and placed in cold saline immediately until the cohesion test was performed (within 2 h after surgery): one piece from the centre of the NAS and a second piece from the AA approximately 2 cm above the sino-tubular junction (STJ). The thickness of both samples was measured immediately before cohesion testing using a micrometer (Kometex B.V./Hogetex, Germany).
Intraoperative echocardiography
Aortic diameters (aortic annulus, AR, STJ and AA dimensions) were assessed by intraoperative transesophageal echocardiography (TOE). TOE was performed with a multiplane 2.9–6.7 MHz (6T-RS) phased-array-probe (Vivid i, GE Healthcare, Milwaukee, WI) prior to cardiopulmonary bypass in all patients.
Aortic wall cohesion testing
The cohesion testing was performed using the Dissectometer ().[Citation9] In brief, the device consists of a mechanical actuator with special customized holding jaws for fixing a sample of the aortic wall from both sides (adventitial and endothelial), thereby simulating the natural mechanism of aortic dissection. The process is recorded simultaneously on digital computer system and converted to tensile strain curves (TSC) ().
Figure 1. Dissectometer and tensile strain curves. (A) Dissectometer: A- actuator, B- camera, C- computer. (B) Tensile strain curve: Localization of the parameters P1–P7; mathematical formula for P8 and P9.
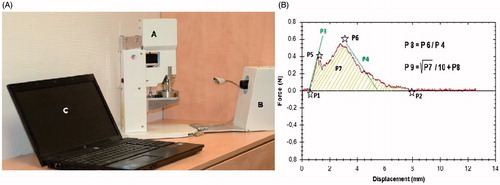
Parameters P1, P2, P5 and P6 correspond to specific points on the tensile strain curve. P1 (mm) is the beginning of the positive deviation – the point when the Dissectometer registers the first tension in the sample. P2 (mm) is the point of the dissection and the power has a value of zero at P2. P5 (N) is the first power maximum (at this point the power has decreased temporarily). After this point the aortic wall sample is damaged irreversibly. P6 (N) represents the “dissection limit” after which the power necessary to disrupt the aorta decreases. P3 (N mm−1) is the angle of the line between P1 and P5. This characteristic describes the elasticity of the aortic wall – the sharper the angle, the higher the elasticity of the aorta. P4 (N mm−1) is the angle of the power decrease, which characterizes the cohesion of the aortic wall. P7 (N mm) represents the area under the TSC, which describes the total cohesion of the aorta. These seven parameters were used to mathematically derive two other parameters: P8 and P9. P8 represents the “dissection tendency” (calculated as the maximal force divided by the downward angle) and P9 represents the “dissection potential” (calculated as the sum of P8 and the square root of P7 divided by 10). The parameters P7, P8 and P9 were found to have the highest predictive value for determining histological aortic wall instability with a positive correlation of test value and quality of the aortic wall.[Citation10] All cohesion tests were performed and analyzed by one observer blinded to all patient data.
Statistical analyses
All descriptive statistics are summarized for categorical variables as frequencies (%). Continuous variables are reported as mean ± standard deviation. For comparison of both groups, the Sign test a distribution-free method for paired samples has been used.
Spearman correlations between all variables were calculated. A value p < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS®, version 19.0 (IBM Corp., Armonk, NY).
Results
Thirty-three adult patients undergoing elective open heart surgery with AR replacement were included in this study. summarizes the patients’ characteristics including age, sex, cardiovascular risk factors and indication for surgery. The mean age of patients was 65 ± 14 years, the majority of patients were male (28/32 (80%)).
Table 1. Demographic variables and comorbidities of patients.
TOE showed a slightly enlarged aorta in all regions (45.3 ± 8.3 mm in AR, 41.9 ± 10.3 mm in the STJ and 46.6 ± 10. 5 mm in the AA).
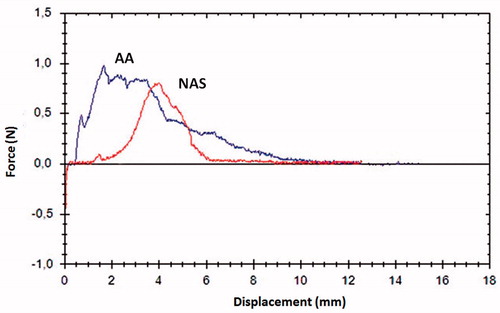
Cohesion testing of the samples with the Dissectometer showed statistically significant differences between the aortic sinus and ascending aorta with a decreased stability for the ascending aorta in all three assessed parameters (P7: 86.0 ± 55.0 vs. 152.3 ± 89.2, p < 0.01; P8: 2.5 ± 1.3 vs. 6.0 ± 3.1, p < 0.01; P9: 3.6 ± 1.4 vs. 7.8 ± 3.2, p < 0.01) (). shows the typical TSC for the AA and NAS with a lower area under the curve for AA representing the total cohesion of the aorta. In addition, it shows impaired elasticity, indicated by the obtuse angle of P3 in the AA sample.
Figure 2. Cohesion testing and AWT of the ascending aorta compared to the NAS. AWT: aortic wall thickness; AA: ascending aorta; NAS: non-coronary sinus.
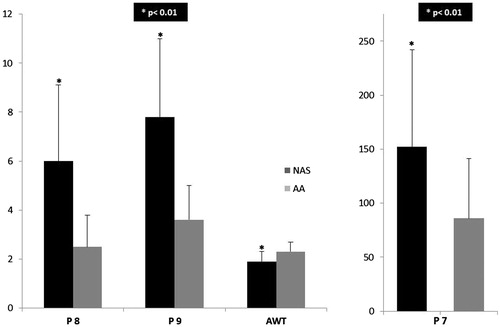
A statistical significant difference in AWTs between both segments with a thinner wall of the NAS could be observed (1.9 ± 0.4 mm vs. 2.3 ± 0.4 mm, p < 0.01). The NAS wall was thinner than the wall of the region of the AA in all patients except for one.
Whereas a positive correlation between the diameter of the STJ and the AWT was found, no association between the dimension of the other aortic regions and the AWT could be observed. In addition, the aortic dimensions did not show any correlation to the aortic wall stability in cohesion testing ().
Table 2. Transesophageal dimensions and correlations with AWT and cohesion.
In contrast, the parameter P8 of the Dissectometer showed a statistically significant correlation to the wall thickness of NAS (CI −0.35, p < 0.05) and AA (CI −0.32, p < 0.05). The correlation of AWT and P7 and P9 tended to be significant but did not reach the statistical cut-off ().
Table 3. Correlation between aortic wall thickness and cohesion testing.
Discussion
Acute dissection of the thoracic aorta is a catastrophic event with an incidence of 0.5–1.0 per 100,000 per year being associated with high mortality and morbidity.[Citation1] In cases of sudden death of non-hospitalized patients, aortic dissection can be proved in 1.5% of necropsied cases.[Citation11] Risk factors for aortic dissection include male sex, older age and prior cardiac surgery, especially aortic valve replacement and aortic manipulation (including angiography and stenting). In addition, acute hemodynamic stress such as that encountered with cocaine use, pheochromocytoma and heavy weightlifting has been associated with aortic dissection.[Citation12] Moreover, certain genetic diseases increase the risk of suffering from an aortic dissection, including Marfan, Ehlers–Danlos and Loeys–Dietz syndromes.[Citation13–15]
The primary tear of the AD is most frequently located in the area of the ascending aorta with equal distribution between the proximal, middle and distal part.[Citation16] The outcome of AD mainly depends on extend of spreading of the dissection from the primary entry with consecutive malperfusion of the heart, brain, intestinal organs or lower extremities. The spreading of the dissection follows the direction of blood flow toward the aortic arch in most of the cases. Retrograde expansion of the dissection involving the AR is associated with a very poor prognosis, sometimes leading to coronary ischemia, acute aortic regurgitation and pericardial tamponade. Currently, it is quite unknown why the primary tear typically arises in the ascending aorta and why the dissection spreads distally in most of the cases.
Some authors hypothesize that histological characteristics and mechanical properties differ significantly between various sections of the aorta: Wu et al. described differences in biological development of the ascending aorta and aortic sinus as potential explanation for the predisposition of ascending aorta for dissection.[Citation8] Whereas the ascending aorta is composed of both cardiogenic mesodermal cells and cardiac neural crest cells, the vascular smooth muscle cells in the root arise from cardiogenic mesoderm cells. Wu et al. hypothesized that the transition points between different vascular smooth muscle cells may represent a focal point of vulnerability for dissection.[Citation8]
In addition to structural characteristics of the aortic wall, movement of the AR during the cardiac cycle might be a key mechanism in the pathogenesis of aortic dissection: Analyses of aortography in 40 patients with suspected coronary artery disease demonstrated that AR motion has a direct impact on mechanical stress of the aorta; the largest stress increase due to AR displacement was found approximately 2 cm above the STJ.[Citation18] The longitudinal stress was found to increase significantly in the ascending aorta above the STJ possibly explaining the high prevalence of circumferential intimal tears and aortic dissections along the greater curvature of the aorta, a few centimeters above the aortic valve.[Citation17]
Della Corte et al. examined 133 adult patients with bicuspid aortic valve using echocardiography over a mean period of 4.5 years to identify risk factors for aneurysm growth.[Citation18] Although an AR phenotype (dilation predominantly at the aortic sinuses, with a normal or less dilated ascending aorta) was identified as an independent predictor for a fast aneurysm growth defined as >0.9 mm/year, baseline aortic dimensions were not associated with further aneurysm expansion.[Citation18] In addition, the most common ascending phenotype was shown to be more stable with slower disease progression.
Even within the ascending part of the aorta, aortic wall properties seem to be heterogeneous: whereas, histology did not show significant differences between regions of the ascending aorta, tensile strain testing revealed the lateral quadrant (outer curvature) to be the stiffest region and the medial quadrant (inner curvature) to be more elastic.[Citation5]
Taken together, there is a growing body of evidence supporting the hypothesis that differences in regional aortic segments might be responsible for the typical pattern of dissection progression. Therefore, this study was performed to describe the regional quality of the aortic wall comparing the ascending aortic and aortic sinus segment with the Dissectometer. Our results clearly show that the stability and wall cohesion of the aortic sinus are statistically significantly greater than those of the ascending aorta. In agreement with a previous publication from our group, this study shows an inverse association between AWT and histological properties of the aorta. A thin aorta seems to be more stable, whereas, thick walls are associated with an impaired cohesion.
These findings may at least partly explain the differences between the mechanical properties of the AA and the NAS: The ascending aorta with its thicker wall may itself represent a weak point with less stability and resistance. It seems to be reasonable to assume that not only one characteristic but also the interaction or sum of all the differences described – histology, function, stability, resistance and hemodynamic influences – between aortic sinuses and the ascending aorta may lead to the typical pattern of AD with the primary entry in the region of the ascending aorta and anterograde expansion in direction to the aortic arch.
Limitations
There are some limitations of our study. First of all, our study suffers from the general limitation of being a single-center, retrospective investigation. Second, the power to detect a difference between the groups was limited due to the small number of patients.
Third, all data reported in this manuscript were based on tissue data from ex vivo tests. However, isolating samples may introduce as yet unknown changes to their behavior affecting the results of in vitro/ex vivo tests.
Conclusions
This study shows that the wall of the thoracic aorta is characterized by a thinner but more stable wall in the AR; whereas, the ascending aortic wall (AA) was found to be weaker despite its higher thickness in TSC analyses.
The observed differences in stability between the aortic sinus and the ascending aorta walls might play an important role in the pathophysiological mechanisms involved during development of the primary dissection tear and the spreading of the dissection toward the AR. However, further studies are necessary to test this potentially causal relationship.
Acknowledgments
We would like to thank Michael Hanna, PhD, for proof-reading the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903.
- O'Gara PT, DeSanctis RW. Acute aortic dissection and its variants. Toward a common diagnostic and therapeutic approach. Circulation. 1995;92:1376–1378.
- Roberts W. Aortic dissection: anatomy, consequences and causes. Am Heart J. 1991;101:195–214.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369.
- Choudhury N, Bouchot O, Rouleau L, et al. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc Pathol. 2009;18:83–91.
- MacLean NF, Dudek NL, Roach MR. The role of radial elastic properties in the development of aortic dissections. J Vasc Surg. 1999;29:703–710.
- Nathan DP, Xu C, Gorman JH 3rd, et al. Pathogenesis of acute aortic dissection: a finite element stress analysis. Ann Thorac Surg. 2011;91:458–463.
- Wu D, Shen YH, Russell L, et al. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–924.
- Benedik J Jr., Azhari P, Tsagakis K, et al. Dissectometer – a new device for tensile strength testing of the vascular wall. Minim Invasive Ther Allied Technol. 2012;21:329–334.
- Pilarczyk K, Tsagakis K, Thielmann M, et al. Detection of aortic wall instability with the new dissectometer: correlation with histological findings. Minim Invasive Ther Allied Technol. 2015;24:233–241.
- Loire R, Tabib A. Mort subite cardiaque inattendue: bilan de 1.000 autopsies. Arch Mal Coeur Vaiss. 1996;89:13–18.
- Stanger O, Schachner T, Gahl B, et al. Circulation. 2013;128:1602–1611.
- Aalberts JJ, van den Berg MP, Bergman JE, et al. The many faces of aggressive aortic pathology: Loeys-Dietz syndrome. Neth Heart J. 2008;16:299–304.
- Bondy CA. Aortic dissection in Turner syndrome. Curr Opin Cardiol. 2008;23:519–526.
- Shalhub S, Black JH 3rd, Cecchi AC, et al. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg. 2014;60:160–169.
- Chen K, Varon J, Wenker OC, et al. Acute thoracic aortic dissection: the basics. J Emerg Med. 1997;15:859–867.
- Beller C, Labrosse MR, Thubrikar MJ, et al. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation. 2004;109:763–769.
- Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1301–1310.