Abstract
Objectives. We investigated whether comorbidity burden of comatose survivors of out-of-hospital cardiac arrest (OHCA) affects outcome and if comorbidity modifies the effect of target temperature management (TTM) on final outcome. Design. The TTM trial randomized 939 patients to 24 h of TTM at either 33 or 36 °C with no difference regarding mortality and neurological outcome. This post-hoc study of the TTM-trial formed a modified comorbidity index (mCI), based on available comorbidities from the Charlson comorbidity index (CCI). Results. Bystander cardiopulmonary resuscitation (CPR) decreased with higher comorbidity group, p = 0.01. Comorbidity groups were univariately associated with higher mortality compared to mCI0 (HRmCI1: 1.55, CI: 1.25–1.93, p < 0.001, HRmCI2: 2.01, CI: 1.55–2.62, p < 0.001, HRmCI ≥ 3: 2.16, CI: 1.57–2.97, p < 0.001). When adjusting for confounders there was a consistent, nonsignificant association between level of comorbidity and mortality (HRmC11: 1.17, CI: 0.92–1.48, p = 0.21, HRmCI2: 1.28, CI: 0.96–1.71, p = 0.10, HRmCI ≥ 3: 1.37, CI: 0.97–1.95, p = 0.08). There was no interaction between comorbidity burden and level of TTM on outcome, p = 0.61. Conclusion. Comorbidity burden was associated with higher mortality following OHCA, but when adjusting for confounders, the influence was no longer significant. The association between mCI and mortality was not modified by TTM. Comorbidity burden is associated with lower rates of bystander cardiopulmonary resuscitation after OHCA.
Introduction
Preexisting comorbidity is associated with worse outcome after out-of-hospital cardiac arrest (OHCA) with higher mortality.[Citation1–3] Others have suggested that comorbidity alone is not associated with higher mortality, when taking confounders into account.[Citation4] However, it seems unclear whether patients with higher comorbidity burden have a different frequency of shockable rhythm, location of arrest, witnessed arrest and bystander cardiopulmonary resuscitation (CPR).
As resuscitation rates have increased,[Citation5] the frequency of successfully resuscitated patients with severe comorbidity may also increase. It is not known whether patients with a higher comorbidity burden receive the same post resuscitation care (neurological prognostication, target temperature management (TTM) and/or revascularization) as patients without or with a low comorbidity burden, and the benefit of theses interventions may differ based on previous illnesses. This has been exemplified in patients categorized by the Pittsburgh cardiac arrest category score (PCAC),[Citation6] as only the patients with the highest comorbidity burden (PCAC IV) did not benefit from acute coronary angiography (CAG).[Citation7] The PCAC is a validated score that takes cardiovascular dysfunction and neurological evaulations into account.
Finally, it is not known whether the effect of TTM is different in patients with different degrees of comorbidity. This sub-study of the TTM-trial aims to: (a) investigate the association between increasing comorbidity burden and mortality in comatose patients resuscitated from cardiac arrest and (b) examine whether a high comorbidity burden modifies the effect of TTM at 33 °C vs. 36 °C on mortality.
Methods
Study design
This is a sub-study of the TTM-trial, which previously included 950 comatose OHCA patients with return of spontaneous circulation (ROSC) admitted to 36 intensive care units (ICUs) in Europe and Australia during 2010–2013.[Citation8] The 950 patients were randomized 1:1 to either 33 or 36 °C.[Citation9] Blinded neurological evaluation was performed 108 h after arrest or later, and active treatment was continued until evaluation had been performed.[Citation10] Of 950 patients included, four withdrew consent and seven were excluded from the modified intention-to-treat population, leaving 939 patients for analyses of mortality and neurological outcome.[Citation10] Pre-hospital data were collected in accordance with the Utstein guidelines.[Citation11]
Ethical approval
The ethical committees in all participating countries approved the TTM protocol. The trial is registered at ClinicalTrials.gov (Identifier: NCT01020916). Informed consent was waived or acquired from all patients or their legal guardians as required by national legislation.[Citation8]
Comorbidity index
In order to grade the level of comorbidity we formed a modified comorbidity index (mCI), inspired by the Charlson Comorbidity Index (CCI). The CCI is a widely used, validated comorbidity index, that weights presence of 22 conditions to predict mortality.[Citation12]
As we did not have all 22 diseases recorded, we used available data to form an index. This modified index consists of patients with no comorbidities (mCI0), patients with one comorbidity recorded (mCI1), patients with two recorded comorbidities (mCI2) and patients with three or more comorbidities (mCI ≥3). The following led to one point in our mCI: Previous AMI/ischemic heart disease/PCI/CABG, diabetes/insulin dependency within diabetes, congestive heart failure, TIA/stroke, asthma/COPD and cirrhosis, while hematological or other malignancy and chronic dialysis led to two points, and AIDS led to six points. Points were added to each other, so that a patient with previous AMI and AIDS would score seven points and thus be placed in the mCI ≥3. Peripheral vascular disease, connective tissue disease, ulcer, hemiplegia, moderate or severe liver disease, malignant tumor with metastasis and diabetes with end organ damage were not recorded in our database, while dementia to some extent may be excluded, as patients with CPC 3–4 prior to arrest were excluded in the study. We validated the modified index in a different population of 798 OHCA patients from the greater Copenhagen area, in which all comorbidities needed for CCI were recorded. All patients in this population had presumed cardiac etiology of arrest.[Citation13] The mCI and CCI demonstrated substantial agreement with unweighted kappa statistic = 0.74.
Outcome
Vital status six months after randomization was obtained from public registry, hospital registry or contact with patient, relatives or primary care physician. Neurological outcome was assessed in patients alive 180 d after OHCA, using the cerebral performance category (CPC) [Citation14] as well as the modified Rankin scale (mRS).[Citation8] The CPC scale divides neurological outcome into 5 categories, where 1–2 are considered favorable outcome, 3–4 are unfavorable and 5 is death. The mRS starts at 0, and 0–3 are considered favorable, while 4–5 are unfavorable and 6 is death.
Statistical analyses
Data are presented as mean standard deviation (SD) for normally distributed data, median (25–75 quartile) for non-normally distributed data and n(%) for categorical data. Differences between comorbidity groups were tested with the Kruskal–Wallis test for characteristics with more than two categories and χ2 test for characteristics with two categories. Numerical differences were tested using the Cochran–Armitage trend test.
Survival until end of study was assessed using the Cox regression adjusting for confounders and unadjusted Kaplan–Meier plots, and compared with log-rank tests. Hazard ratios (HR) with 95% confidence intervals (CI) are reported. Whether comorbidity modified the effect of TTM on mortality was assessed by including an interaction in the multiple Cox regression.
Neurological outcome (favorable vs. unfavorable outcome) at 180 d and utilization of CAG and percutaneous coronary intervention (PCI) was assessed using logistic regression on, adjusting for confounders. Odds ratios (OR) and 95%CI were reported. All analyses were performed in R.3.01.[Citation15]
Results
Age increased with mCI groups, as patients with mCI0 had a mean age of 60 years compared to 68 years in mCI1 and 70 years in patients with mCI2 and mCI ≥3, p < 0.001, . There was a different distribution of males throughout comorbidity groups (79% vs. 87%, 80% and 78% in mCI0, mCI1, mCI2 and mCI ≥3, respectively, p = 0.04). Despite equal distribution of witnessed arrest and arrest in public places, fewer bystanders performed CPR in higher comorbidity groups (76% in mCI0 vs. 62% in mCI ≥3, p = 0.01). Comorbidity burden was not significantly associated with lower bystander CPR in multiple regression, ORmCI1: 0.90, CI: 0.63–1.28, p = 0.54, ORmCI2: 0.69, CI: 0.44–1.08, p = 0.10, ORmCI ≥ 3: 0.59, CI: 0.34–1.02, p = 0.06. Shockable rhythm was less frequent in higher comorbidity groups (83% in mCI0 vs. 65% in mCI ≥3, p = 0.001) and STEMI was less frequent (50% in mCI0 vs. 27% in mCI ≥3, p < 0.001).
Table 1. Baseline demographic, pre-hospital characteristics and post resuscitation care treatment in the four comorbidity groups.
Post-resuscitation care
CAG and PCI was performed less frequently in higher comorbidity groups (CAG: mCI0: 73%, mCI1: 59%, mCI2: 45%, mCI ≥3: 53%, and PCI: mCI0: 52%, mCI1: 35%, mCI2: 26%, mCI ≥3: 19%, both: p < 0.001, ).
Comorbidity groups mCI1 and mCI ≥3 were not significantly associated with lower utilization of CAG <24 h post ROSC in multiple logistric regression adjusting for comorbidity, sex, primary rhythm, location of arrest, witnessed arrest, STEMI, age, time to ROSC and bystander CPR, but mCI2 was (ORmCI2: 0.48, CI: 0.30–0.76, p = 0.02).
For PCI after CAG, there was only a significant association between lower utilization of PCI in the mCI3 group (ORmCI ≥ 3: 0.33, CI: 0.14–0.75, p = 0.01).
Mortality and neurological outcome
In univariate analysis, mCI1, mCI2 and mCI3 were all significantly associated with higher mortality after end of study (HRmCI1: 1.55, CI: 1.25–1.93, p < 0.001, HRmCI2: 2.01, CI: 1.55–2.62, p < 0.001, HRmCI≥: 32.16, CI: 1.57–2.97, p < 0.001, ).
Figure 1. Kaplan–Meier plot of survival at end of study in comorbidity groups. Differences were tested with log-rank tests. mCI: modified comorbidity index.
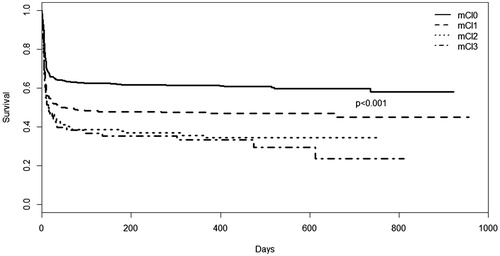
When adjusting for age at arrest, sex, primary rhythm, location of arrest, witnessed arrest, bystander CPR, bystander defibrillation, time to ROSC, type of cooling device, STEMI, level of TTM, CAG <24 h post ROSC and lactate level at admission, there was no longer any significant association between any level of comorbidity and higher mortality at end of study.
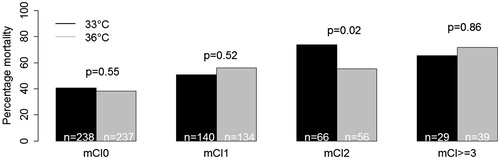
We did not detect any interaction between level of TTM and comorbidity index in multiple analysis (mCI0: p: 0.86, mCI2: p: 0.10, mCI ≥3: p: 0.67), or between TTM stepwise increase in comorbidity index, p: 0.61. Log-rank test in each mCI group did not reveal differences between the two temperatures, except in mCI2 at p = 0.02, .
Figure 2. Mortality at temperatures 33 °C and 36 °C in the four comorbidity groups. Differences were tested with log-rank test. There was no significant interaction between mortality and target temperature in multiple Cox regression (p = 0.61).
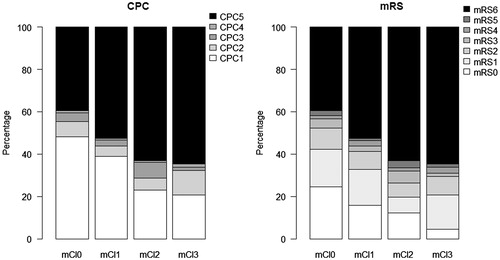
Neurological outcome differed significantly among comorbidity groups for CPC as well as mRS, p < 0.001, . This was still significantly different when dichotomizing into favorable (CPC 1–2, mRS 0–3) vs. unfavorable outcome (CPC 3–5, mRS 4–6) at p < 0.001, but when limiting the analysis to survivors there was no difference in mRS (favorable outcome: mCI0: 94%, mCI1: 92%, mCI2: 87%, mCI ≥3: 88%, p = 0.29), but CPC was still statististically significant (favorable outcome: mCI: 92%, mCI1: 92%, mCI2: 78%, mCI ≥3: 92%, p = 0.02).
Figure 3. Neurological outcome in the four comorbidity groups by CPC and mRS 180 d after hospital discharge. There were no significant differences in favorable neurological outcome in survivors when adjusting for confounders.
In multiple logistic regression, mCI groups were not significantly associated with unfavorable outcome in CPC (ORmCI1: 0.81, CI: 0.35–1.79, p = 0.61, ORmCI2: 2.21, CI: 0.85–5.39, p = 0.09, ORmCI ≥ 3: 0.74, CI: 0.11–3.01, p = 0.71) and the same was true for mRS (ORmCI1: 1.02, CI: 0.42–2.37, p = 0.97, ORmCI2: 1.61, CI: 0.52–4.47, p = 0.38, ORmCI3: 1.44, CI: 0.30– 5.22, p = 0.61), when adjusting for sex, age, primary rhythm, location of arrest, witnessed arrest, STEMI, time to ROSC and bystander CPR.
Discussion
In this study we found that comorbidity was associated with higher mortality in simple analysis, but this association was no longer significant after adjustment for demography and cardiac arrest characteristics. Higher comorbidity burden was not significantly associated with higher odds of unfavorable neurological outcome. Comorbidity was not associated with lower utilization of post resuscitation care, suggesting that other factors than comorbidity are important when it comes to mortality, neurological outcome and post resuscitation care after OHCA.
Weighting and indexing comorbidities
Since the index used in this study is a modified version of the CCI, it suffers from many of the same advantages and disadvantages. Furthermore, our index is limited by not taking all conditions used in the CCI into account, but it does show a good relation to the CCI. As many other comorbidity indices, the CCI does not take into account different combinations of comorbidities.[Citation16] Compared to other indices, the CCI has a low generalizability, as the conditions used in the original index (predicting short term mortality in a general hospital ward) may not be generilizable to predict mortality in all other settings, but the content validity (completeness and relevance of the content related to what it claims to measure) is high.[Citation16] In addition, a recent comparison of comorbidity indices found that the CCI is a valid method for comparing disease severity, if the outcome is mortality.[Citation17] The CCI is also the most comprehensively studied comorbidity index to assess mortality, and thus we chose it as a basis for our index.[Citation17] The approximations used, for example in our category of “Previous AMI, ischemic heart disease, previous PCI, previous CABG” can be discussed, as this does not take into account that these conditions may not be similar in severity and may be found in different combinations. However, we have chosen this approach to ensure that all indications of previous ischemic disease were weighted in our index.
Bystander CPR and comorbidity
Patients with higher comorbidity scores had lower rates of bystander CPR, even though the presence of witnessed arrest and location of arrest did not differ. To our knowlegde, it is unknown if patients with higher comorbidity burden in general have different characteristics at arrest than patients with lower scores. Our study may indicate that bystanders are less likely to iniate CPR, but it is not clear whether age, frailty and relation to the OHCA victim of the bystander influenced the decision to perform or not perform CPR. In our study, age and location of arrest were significantly associated with utilization of CPR, but we cannot determine how frailty and age interact. However, as CPR may be a marker of an arrest “where action is taken” and prevent a shockable rhythm from deteriorating into non-shockable rhythm,[Citation18,Citation19] assessing whether comorbidity and age influenced the decision to perform CPR or not may be worthwhile.
The assocation between comorbidity, mortality and neurological outcome
In general, it is expected that the comorbidity burden increases with age,[Citation20] but a recent review found little evidence on the predictive value on comorbidities in elderly.[Citation20] Others do not find a significant association between CCI and favorable neurological outcome, and suggest that age rather than comorbidity is an independent marker of prognosis.[Citation4] This is further supported by a study that evaluated the relation between comorbidites assessed by CCI prior to OHCA and neurological outcome in elderly ≥70, that did not find a relation between comorbidity and neurological outcome.[Citation21] It should be noted that the CCI is originally formed to predict mortality and not functional outcome. Therefore, our study focused on mortality primarily and neurological outcome secondarily, but we found a similar pattern, that age remained significantly associated with mortality when adjusting for confounders, while comorbidity did not. With regards to neurological outcome, we found no difference between comorbidity groups in survivors as measured by mRS, but for CPC a significant difference was found. However, comorbidity groups were not significantly associated with neurological outcome when adjusting for confounders, which may support the notion that comorbidity did not predict neurological outcome. As this study focused on patients who had successfully been resuscitated, we cannot rule out that patients with higher comorbidity scores would have a lower chance of successful resuscitation, and thereby creating a selection bias in our cohort.
Post-resuscitation care and comorbidities
When deciding whether to perform CAG in patients with comorbidities, the risk of contrast-induced renal failure and other complications after CAG/PCI are taken into account. As these complications may increase with age,[Citation22] elderly patients and patients with a higher comorbidity burden may receive fewer interventions. In patients with acute myocardial infarction revascularization was less utilized in higher PCAC comorbidity scores, even when excluding patients with known renal failure.[Citation23] This did not take comorbidities that were not considered in PCAC into account, but it did highlight the challenging decision-making regarding post-resuscitation care in patients with a higher comorbidity burden. We did not find a consistent pattern related to utilization of CAG/PCI, as only one group (mCI2) received significantly fewer CAG when adjusting for confounders. This may suggest that comorbidities are not the most important factor when deciding whether a patient is eligible for CAG/PCI or not. At present it may be, that age and clinical presentation affects decision-making regarding invasive procedures to a greater extend than comorbidities. However, it may be difficult to determine the causal relation as each case was decided at the descretion of the treating physician based on all available information.
Limitations
It is a limitation to our study that we did not have all relevant comorbidities available for creating a complete CCI.
Conclusion
Comorbidity was not significantly associated with neither mortality nor neurological outcome when taking demography and cardiac arrest characteristics into account. Neither did comorbidity modify the effect of level of TTM, nor interacted with age.
Disclosure statement
MSc. Winther-Jensen, Drs. Thomsen, Søholm, Frydland, Kjaergaard and Kuiper report no conflicts of interest.
Funding
Dr. Friberg reports lecture fees from BARD Medical and NatusInc., outside the submitted work. Dr. Nielsen reports Speakers Honorarium for BARD Medical, outside the submitted work. Dr. Hassager reports lecture fees from Novartis, TEVA and ViCare, outside the submitted work.
References
- Nolan JP, Laver SR, Welch CA, et al. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC case mix programme database. Anaesthesia. 2007;62:1207–1216.
- Herlitz J, Bång A, Gunnarsson J, et al. Factors associated with survival to hospital discharge among patients hospitalised alive after out of hospital cardiac arrest: change in outcome over 20 years in the community of Göteborg, Sweden. Heart. 2003;89:25–30.
- Iqbal MB, Al-hussaini A, Rosser G, et al. Predictors of survival and favorable functional outcomes after an out-of-hospital cardiac arrest in patients systematically brought to a dedicated heart attack center (from the Harefield cardiac arrest study). Am J Cardiol. 2015;115:730–737.
- Terman SW, Shields TA, Hume B, et al. The influence of age and chronic medical conditions on neurological outcomes in out of hospital cardiac arrest. Resuscitation. 2015;89:169–176.
- Wissenberg M, Lippert FK, Folke F, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310:1377–1384.
- Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82:1399–1404.
- Reynolds JC, Rittenberger JC, Toma C, et al. Risk-adjusted outcome prediction with initial post-cardiac arrest illness severity: implications for cardiac arrest survivors being considered for early invasive strategy. Resuscitation. 2014;85:1232–1239.
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med. 2013;369:2197–2206.
- Nielsen N, Hovdenes J, Bishop G, et al. Target temperature management after out-of-hospital cardiac arrest-a randomized, parallel-group, assessor-blinded clinical trial-rationale and design. Am Heart J. 2012;163:541–548.
- Nielsen N, Wetterslev J, Cronberg T, et al. Supplement to: Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206.
- Langhelle A, Nolan J, Herlitz J, et al. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: the Utstein style. Resuscitation. 2005;66:271–283.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Winther-Jensen M, Kjaergaard J, Hassager C, et al. Resuscitation and post resuscitation care of the very old after out-of-hospital cardiac arrest is worthwhile. Int J Cardiol. 2015;201:616–623.
- Brain Resuscitation Clinical Trial I Study Group (BRCT-1). Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med. 1986;314:397–403.
- R Foundation for Statistical Computing. R.3.01, R Core Team [Internet]. Vienna 2013. p. http://www.R–project.org/. Available from: http://www.r-project.org.
- Hall SF. A user’s guide to selecting a comorbidity index for clinical research. J Clin Epidemiol. 2006;59:849–855.
- De Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–229.
- Hasselqvist-Ax I, Riva G, Herlitz J, et al. Early cardiopulmonary resuscitation in out-of-hospital cardiac arrest. N Engl J Med. 2015;372:2307–2315.
- Waalewijn RA, Nijpels MA, Tijssen JG, et al. Prevention of deterioration of ventricular fibrillation by basic life support during out-of-hospital cardiac arrest. Resuscitation. 2002;54:31–36.
- van de Glind EM, van Munster BC, van de Wetering FT, et al. Pre-arrest predictors of survival after resuscitation from out-of-hospital cardiac arrest in the elderly a systematic review. BMC Geriatr. 2013;13:68.
- Beesems SG, Blom MT, van der Pas MH, et al. Comorbidity and favorable neurologic outcome after out-of-hospital cardiac arrest in patients of 70 years and older. Resuscitation. 2015;94:33–39.
- Sidhu RB, Brown JR, Robb JF, et al. Interaction of gender and age on post cardiac catheterization contrast-induced acute kidney injury. Am J Cardiol. 2008;102:1482–1486.
- Balzi D, Barchielli A, Buiatti E, et al. Effect of comorbidity on coronary reperfusion strategy and long-term mortality after acute myocardial infarction. Am Heart J. 2006;151:1101–1107.
