Abstract
Although, treatment of ischemic heart disease (IHD) has improved considerably within the last decades, it is still the main cause of death worldwide. Despite maximum treatment, many IHD patients suffer from refractory angina and heart failure, which severely limits their daily lives. Moreover, IHD is very costly for the health care system. Therefore, new treatment options and strategies are being researched intensely. Stem cell therapy to improve myocardial perfusion and stimulate growth of new cardiomyocytes could be a new way to go. Nevertheless, the results from clinical studies have varied considerably, probably due to the use of many different cell lines obtained from different tissues and the different patient populations. The present review will focus on treatment with the mesenchymal stromal cell from bone marrow and adipose tissue in animal and patients with acute and chronic IHD (CIHD).
Introduction
Ischemic heart disease (IHD) causes 40% of all deaths in the European Union (EU) alone and is the main cause of death in both women and men. (ehnheart.org/cvd-statistics.html) The overall cost of IHD in EU is almost €196 billion a year. Advances in conventional therapies have reduced the mortality of IHD significantly, but have also left an increasing number of patients with symptomatic chronic IHD (CIHD) in spite of these therapies.[Citation1] These patients suffer from daily chest pain without any further revascularization options (refractory angina), dyspnea caused by heart failure and reduced quality of life. An increasing morbidity rate of this nature in an ageing population is a huge burden for society. Besides the expenses related to hospitalization, repetitive invasive and non-invasive procedure, there are a loss in national income related to e.g. sick pay and early retirement.
Therefore, there are unmet needs for novel, effective treatments of CIHD to improve patient survival rates, employment, quality of life and reduce health care costs. A promising such new therapeutic concept is stem cell therapy.[Citation2]
Various mononuclear cell populations obtained from bone marrow have been widely used for regenerative purposes in clinical trials in patients with IHD.[Citation3–12] Mononuclear cells from the bone marrow consists of both hematopoietic and mesenchymal stromal cells (MSCs) in addition to monocytes and lymphocytes. Different subpopulations of cells or single cell lines from the bone marrow have been tested clinically, e.g. mononuclear cells, CD34+ cells, CD133+ cells, MSCs, etc., and so far no consensus exists about the best cell type for the treatment.
The presence of MSCs in most tissues of the body indicates their importance and pre-clinical studies also designate that their regenerative capacity is comparable regardless of tissue origin. For clinical use most studies have used bone marrow derived MSCs (BMSCs). However, recently focus has moved toward stromal cells from other tissues and especially the adipose tissue derived stromal cells (ASCs) as a source for cell therapy.
The aim of this review is to outline the present knowledge about MSC and ASC therapy in animal models and patients with IHD.
Mesenchymal stromal cells
MSCs for clinical studies are presently isolated from several different tissues, such as bone marrow, adipose tissue, cardiac tissue and umbilical cord tissue.[Citation13] Although there are minor differences in cell characteristics, the different cell lines all fulfill the overall defined criteria for being a MSC.[Citation14] Whether they have identical regenerative properties remains to be tested clinically. The predominant hypothesis about the regenerative properties exerted by MSCs is that paracrine mechanisms, which means secretion of a secretome, stimulate reconstruction mediated by surrounding cells. The mechanisms of actions are outlined in .[Citation15]
Figure 1. Mesenchymal stromal cells – mechanisms of action. (Reprint from Singer NG and Caplan AL. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011; 6:457–78. With permission from Annual Reviews).
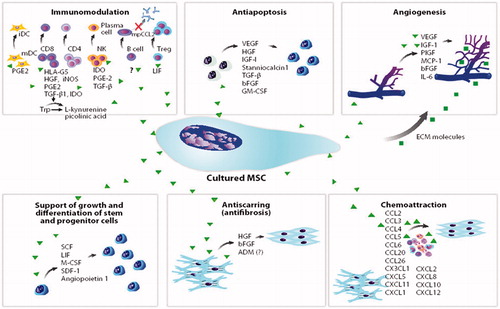
The vast number of similarities shared by MSCs from various tissue sources is not just restricted to morphology, kinetics, surface markers and multipotency, but also related to a shared ability to influence the immune system.[Citation16] MSCs are unique as they possess regenerative as well as immunosuppressive abilities, by which they evade being recognized and rejected by a recipient immune system. These immunosuppressive capabilities allow ASCs to be used for allogeneic therapy, as it offers the promise of an “off-the-shelf” product much like conventional types of medicines. MSCs express only low levels of MHC class I (HLA-ABC), while expressing no MHC class II (HLA-DR/DP/DQ) or co-stimulatory molecules, thereby making them less likely to interact with immune cells.[Citation17] Besides direct cell-cell interaction with antigen-presenting cells, MSCs secrete a number of factors modulating the local environment ().[Citation15]
Even though the immunomodulatory mechanisms are shared by MSCs of any tissue origin, a comparison between BMSCs and ASCs revealed a higher level of cytokine secretion from ASCs, which may indicate that ASCs have a more effective immunomodulatory capacity.[Citation18] These immunological properties provide a strong base for the therapeutic potential of MSCs, not only in an autologous setting but also for allogeneic treatment.
An important aspect of MSC therapy is that the cells have to be culture expanded to reach a sufficient number for clinical therapy. Therefore, it can be an advantage, from a feasibility point of view, that ASCs grow faster than BMSCs during culture expansion. And also a higher yield of ASCs can be isolated from abdominal adipose tissue compared to BMSCs from bone marrow. Allogeneic MSCs and ASCs have been used in several clinical trials without any side-effects.[Citation19–22]
Animal studies with stem cell therapy for CIHD
Animal studies are used to evaluate mode of action, the safety profile and potentially efficacy of new therapies. However, it can be challenging to transfer data obtained in animal studies into human studies due to difficulties with establishing identical disease models, differences between animal species and the xenogeneic nature of a human stem cell product if tested in an animal. Therefore, it is important to get an overview of results obtained in different animal studies.
A large meta-analysis collected data from 52 publications with more than 800 animals treated with stem cells.[Citation23] The animals were treated with many different cell lines, such as bone marrow mononuclear cells, skeletal myoblasts, BMSCs, endothelial progenitor cells, cardiosphere derived cells, embryonic stem cells, hematopoietic progenitor cells and ASCs. Different routes for cell delivery were also used in studies of acute myocardial infarction (AMI) (n = 23) and CIHD (n = 29). The meta-analysis demonstrated that stem cell therapy significantly increased left ventricular ejection fraction (LVEF) by 7.5% compared to controls. The analysis also indicated that the highest benefit was related to: (1) high number of cells, (2) CIHD (vs. AMI), (3) infarction located in the left anterior descending artery and (4) greater degree of ischemic myocardium involved. Moreover, an interesting sub-analysis demonstrated that animals treated with MSCs had a better outcome compared to treatment with bone-marrow derived mononuclear cells. The largest study in the meta-analysis included 47 sheep with AMI, which were treated with 25–450 × 106 allogeneic BMSCs.[Citation24] This randomized, dose-escalating, placebo-controlled study indicated that intra-myocardial delivery of BMSCs may reduce post-AMI left ventricular remodeling in a dose-dependent manner. Interestingly, another meta-analysis found no differences in outcome when comparing animals with IHD treated with either autologous or allogeneic stem cells.[Citation25]
Mesenchymal stromal cell for treatment of patients with STEMI
Culture expanded BMSCs have been tested in several studies in patients with ST-elevation myocardial infarction (STEMI) [Citation19,Citation26–29] () The largest was a randomized, placebo-controlled study which included 69 STEMI patients treated with primary percutaneous coronary intervention followed by intracoronary infusion of 48–60 × 109 autologous BMSC (n = 34) or placebo infusion.[Citation26] The study demonstrated at 3 months follow-up that the treatment was safe and with a significant increase in LVEF which persisted at 6 months follow-up. In addition, positron emission tomography measured perfusion defects decreased significantly at 3 months follow-up.
Table 1. Mesenchymal stromal cells in patients with acute myocardial infarction.
In patients presented with acute myocardial infarction, an interesting double blinded, placebo-controlled study is the Prochymal trial, in which 53 patients with STEMI were treated with allogeneic BMSCs intravenously in a dose titrating design (0.5, 1.6 or 5 × 106 allogeneic BMSCs per kg bodyweight) has been published.[Citation19] The study found that patients treated with BMSCs had significantly fewer episodes of cardiac arrhythmia and also forced expiratory volume in 1 second (FEV1) was significantly higher in the group treated with BMSCs at 6 months follow-up. There was no dose dependent effect and identical improvements in LVEF in the two groups. In a sub-analysis, it was demonstrated that in patients with an anterior STEMI, the LVEF increased significantly compared to baseline in the BMSC treated group. An identical observation was not found in the control group.
It can be concluded that there seems to be an effect of BMSC treatment on myocardial function in some STEMI patients. However, larger clinical trials are needed to explore the treatment dosage and effects further before it can be recommended as a standard treatment for STEMI.
MSCs in patients with CIHD
Several studies have investigated the effect of MSCs in CIHD patients with and without heart failure (). The first larger clinical safety and efficacy trial included 31 patients with severe CIHD and refractory angina.[Citation8] The BMSCs were culture expanded and stimulated with VEGF the last week in culture to drive them toward angiogeneic progenitor cells. The treatment was safe and with clinical effect in a 3 years follow-up period.[Citation9,Citation10] Three years after treatment the patients still had increased working capacity, improved myocardial pumping function, reduced cardiac pain and use of medication, greater quality of life and reduced number of hospitalizations than before treatment (). This study showed a trend toward improved outcome with increasing number of cells injected.
Figure 2. Hospital admission rates per patient from 3 years before treatment to 3 years after treatment. NS: non-significant; PCI: percutane coronary intervention; UAP: unstable angina pectoris; AMI: acute myocardial infarction. (Reprinted from Mathiasen et al. Int J Cardiol. 2013 Dec 10;170(2):246–51) with permission from Elsevier).
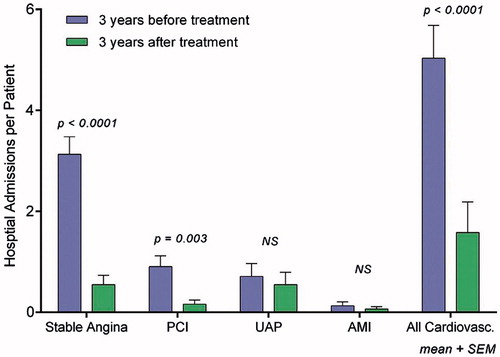
Table 2. Mesenchymal stem cells in patients with chronic ischemic heart disease.
Based on these results a prospective, randomized, double-blind, placebo-controlled phase II study was initiated in patients with CIHD and refractory angina. There was a change in cell population used from BMSC to ASC. The study investigated the effects of direct intra-myocardial injection of VEGF-stimulated ASCs in 60 patients (MyStromalCell Trial).[Citation11] The patients were randomized in a 2:1 ratio to receive ASCs or placebo. The study is finalized but data still not published.
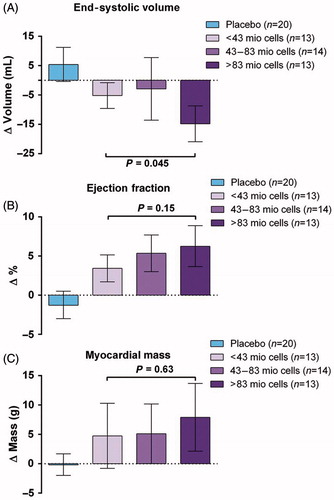
The largest study with BMSC treatment of patients with CIHD and heart failure (MSC-HF Trial) was published in 2014.[Citation12,Citation19] It was a phase II, double-blind, randomized, placebo-controlled trial with direct intra-myocardial injection of autologous BMSC in patients with CIHD and heart failure. A total of 60 patients were randomized in a 2:1 pattern to receive intra-myocardial injections of either BMSCs or placebo. The MSC-HF Trial demonstrated at 6 months follow up significant improvement in the primary endpoint, left ventricular end systolic volume, which was reduced significantly in the BMSC group compared to placebo (). There were also significant improvements in LVEF, stroke volume and myocardial mass between the BMSC and placebo group, but not in scar tissue.[Citation12] Moreover, the treatment was safe and there was a clear tendency toward a dose-response effect with a higher improvement in cardiac function (end-systolic volume and ejection fraction) in patients treated with more than 84 × 106 BMSCs ().
Figure 3. Differences in cardiac function from baseline to 6-month follow-up. (A) End-systolic volume, (B) end-diastolic volume, (C) ejection fraction, (D) stroke volume, (E) cardiac output, (F) myocardial mass, and (G) scar tissue (Paired t-test. Bar values are mean ±95% confidence intervals). (Reprinted from Mathiasen et al. Eur Heart J. 2015 Jul 14;36(27):1744–53 with permission from Oxford University Press).
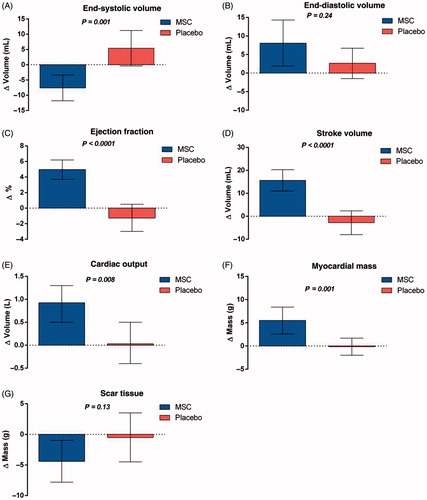
Figure 4. Dose–response effects: (A) end-systolic volume, (B) ejection fraction, and (C) mean myocardial mass. p Values represent the differences between subgroups of the mesenchymal stromal cell group (one-way ANOVA. Bar values are mean ±95% confidence intervals). (Reprinted from Mathiasen et al. Eur Heart J. 2015 Jul 14;36(27):1744–53 with permission from Oxford University Press).
Clinical trials with allogeneic mesenchymal stromal cells
A few clinical trials with allogeneic MSCs have also been conducted ( and ). The first study comparing direct intra-myocardial injection of allogeneic vs. autologous BMSCs in 30 patients with CIHD and heart failure was the POSEIDON Trial.[Citation20] It was a dose-escalating study (20 × 106, 100 × 106 or 200 × 106 BMSCs) without any control group. There was no difference in serious adverse events for up to 1 year follow-up between the two cell populations. Patients who received autologous BMSCs increased performance in 6-min walk tests at 6 and 12 months follow-up significantly compared to baseline, while it was unchanged for patients receiving allogeneic BMSCs. However, both allogeneic and autologous BMSCs reduced infarct size measured with computed tomography scan at 13 months follow-up compared to baseline. No statistical significant increase in LVEF was seen in the groups. Interestingly, an inverse dose–response effect on LVEF, LV systolic volume and infarct size was found for patients receiving 20 × 106 vs. 200 × 106 BMSCs.
Recently, a clinical feasibility and safety trial has been published using bone marrow allogeneic mesenchymal precursor cells (MPCs, 25, 75 or 150 × 106 cells) in patients with CIHD and heart failure.[Citation21] A total of 60 patients were included and divided into three dosing cohorts (20 per dose group) and randomized to trans-endocardial MPC injections (n = 15) or mock procedures (n = 5). The treatment was safe for up to 3 years follow-up with no difference in adverse events between groups. No clinically symptomatic immune responses were noted. There were no differences between MPC-treated and control patients in survival probability, major adverse cardiac events (MACE)-free probability and all-cause mortality. In a post hoc analysis the heart failure related MACE (heart failure hospitalization, successfully resuscitated cardiac death or cardiac death) were significantly reduced in the 150 × 106 MPC group (0/15) vs. control (5/15; 33%), 25 × 106 MPC group (3/15; 20%) and 75 × 106 MPC group (6/15; 40%). This indicates that high-dose allogeneic MPCs may provide benefits in this population.
Discussion
CIHD with and without heart failure is a serious condition with great impact on the patients physical function and quality of life. In addition, it is a very expensive disease with a huge burden on the society due to the increasing number of patients with the disease. Therefore, it is very interesting that treatment with MSCs seems to be effective in improving the heart function and reduce the patient’s symptoms. Some studies also indicate that MSC treatment of CIHD can have an impact on reducing heart failure related MACE and hereby reduce hospitalization and health care costs.
Most clinical studies have used autologous MSCs for clinical therapy and it has proved safety and efficacy as a regenerative treatment for ischemic heart patients with and without heart failure in several clinical trials. One limitation with MSCs is, independent of tissue origin that the cells need to be culture expanded before there is enough cells for a treatment session. The number of cells, which can be isolated from abdominal adipose tissue is higher than from bone marrow and the ASC seems to be more easy to culture expand than BMSCs without going into senescence.[Citation30] Due to the comparability between BMSCs and ASCs, it is expected that the clinical efficacy in the conducted MyStromalCell trial will be comparable with the results of BMSCs treatment in patients with IHD and refractory angina.[Citation8–11]
Safety is always an important part of testing a new treatment modality and it has also been the focus of all MSC studies. Therefore, it is important that there has not been detected any serious stem cell related side effects in any of the MSC/ASC clinical trials independent of whether it has been autologous or allogeneic and regardless of the administration method.
It has been realized that using autologous stem cell populations can be problematic in the disseminating and establishing of such a therapy as a more general treatment option for patients. This is due to the individual variations in cell functionality, cell culture expansion time and amount of cells available for treatment in addition to logistics around transportation and timing of the treatment. To solve these problems and to increase feasibility, repeatability and dissemination of regenerative therapy, a switch from autologous to allogeneic MSC therapy has been considered and established by many research groups and companies. Therefore, it is important that one study already has demonstrated that direct intra-myocardial injection of both allogeneic and autologous BMSCs lead to comparable improvements in cardiac function in patients with CIHD and heart failure.[Citation26]
Several large academia- and company based clinical trials are presently ongoing with allogeneic MSCs isolated and culture expanded from different tissues in patients with CIHD with and without heart failure. However, no comparative studies have yet been conducted to demonstrate or clarify whether one allogeneic cell line is more efficient than another. The MSC treatment dose and delivery method is also not standardized between the studies and can therefore influence the efficacy and outcome. Moreover, the MSCs are living cells, which will behave differently during different isolation and culture expansion conditions.[Citation30] Therefore, variations in efficacy need not to be determined by the actually cell type, but more by the handling of the cells before the treatment. The larger ongoing studies will indicate whether one stem cell concept is superior to the other.
There is intensive research activity in many stem cell supporting commercial companies to improve the many steps from isolation of cells until treatment. This includes research in culture expansion with bioreactor systems, new and better culture media and improving procedures for freezing and storage of the cells for better cell survival. It can therefore, be expected that these initiatives will further improve and optimize stem cell therapy in the future.
In conclusion, there are many clinical data, which indicate that stem cell therapy in CIHD with MSC is safe and seems to be able to improve cardiac function and relieve patient’s symptoms. If these encouraging results are confirmed in the larger ongoing clinical trials, then the time point for MSCs therapy to be implemented in general treatment praxis may not be that far away.
Disclosure statement
None declared.
References
- Kazi DS, Mark DB. The economics of heart failure. Heart Fail Clin. 2013;9:93–106.
- Kastrup J. Stem cells therapy for cardiovascular repair in ischemic heart disease: How to predict and secure optimal outcome? EPMA J. 2011;2:107–117.
- Fisher SA, Doree C, Brunskill SJ, et al. Bone marrow stem cell treatment for ischemic heart disease in patients with no option of revascularization: a systematic review and meta-analysis. PLoS One. 2013;8:e64669.
- Heeger CH, Jaquet K, Thiele H, et al. Percutaneous, transendocardial injection of bone marrow-derived mononuclear cells in heart failure patients following acute ST-elevation myocardial infarction: ALSTER-Stem Cell trial. EuroIntervention. 2012;8:732–742.
- Tendera M, Wojakowski W, Ruzyłło W, et al. REGENT Investigators. Intracoronary infusion of bone marrow-derived selected CD34 + CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT). Trial Eur Heart J. 2009;30:1313–1321.
- Lezaic L, Socan A, Poglajen G, et al. Intracoronary transplantation of CD34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2015;21:145–152.
- Gyöngyösi M, Wojakowski W, Lemarchand P, et al. ACCRUE Investigators. Meta-analysis of cell-based Cardiac studies (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–1360.
- Friis T, Haack-Sørensen M, Mathiasen AB, et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J. 2011;45:161–168.
- Haack-Sørensen M, Friis T, Mathiasen AB, et al. Direct intramyocardial mesenchymal stromal cell injections in patients with severe refractory angina: one-year follow-up. Cell Transplant. 2013;22:521–528.
- Mathiasen AB, Haack-Sørensen M Jørgensen E., et al. Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina-final 3-year follow-up. Int J Cardiol. 2013;170:246–251.
- Qayyum AA, Haack-Sørensen M, Mathiasen AB, et al. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): study design. Regen Med. 2012;7:421–428.
- Mathiasen AB, Qayyum AA, Jørgensen E, et al. Bone-marrow derived mesenchymal stromal cell treatment in patients with severe ischemic heart failure: a randomised placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36:1744–1753.
- Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
- Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–648.
- Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219.
- Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134.
- Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–463.
- TiGenix web page [Internet]. [cited 2016 Sep 09]. Available from: www.tigenix.com.
- Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286.
- Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379.
- Perin EC, Borow KM, Silva GV, et al. A Phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117:576–584.
- De la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323.
- van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658.
- Dixon JA, Gorman RC, Stroud RE, et al. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120:S220–S229.
- Jansen Of Lorkeers SJ, Eding JE, Vesterinen HM, et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circ Res. 2015;116:80–86.
- Katritsis DG, Sotiropoulou PA, Karvouni E, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–329.
- Yang Z, Zhang F, Ma W, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct-relative artery. Cardiovasc Ther. 2010;28:380–385.
- Houtgraaf JH, den Dekker WK, van Dalen BM, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540.
- Mathiasen AB, Jørgensen E, Qayyum AA, et al. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial). Am Heart J. 2012;164:285–291.
- Juhl M, Tratwal J, Follin B, et al. Comparison of clinical grade human platelet lysates for cultivation of mesenchymal stromal cells from bone marrow and adipose tissue. Scand J Clin Lab Invest. 2016;76:93–104.
- Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–556.
- Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–473.
- Katritsis DG, Sotiropoulou P, Giazitzoglou E, et al. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9:167–171.
- Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796.
- Lasala GP, Silva JA, Kusnick BA, et al. Combination stem cell therapy for the treatment of medically refractory coronary ischemia: a Phase I study. Cardiovasc Revasc Med. 2011;12:29–34.
