Abstract
Objectives. The aim of the study was to investigate if adequate preservation of coronary artery endothelium-dependent relaxation and contractility may be obtained after 8 hours of non-ischemic heart preservation. Design. Porcine hearts were perfused for 8 hours at 8 °C, either in cycles of 15 minutes perfusion and 60 minutes non-perfusion, or by continuous perfusion. The perfusate consisted of a cardioplegic, hyperoncotic nutrition solution with oxygenated red cells, and the perfusion pressure was 20 mmHg. In organ baths, coronary artery segments from the preserved hearts were studied and compared to fresh controls. Results. Endothelium-dependent relaxation and contractility were fully preserved after both intermittent and continuous perfusion, as compared to fresh controls. No myocardial edema was seen; water content of the myocardium was 79.5 ± 0.2%, 79.0 ± 0.4% and 79.0 ± 0.3% (ns) for fresh controls, intermittently perfused, and continuously perfused hearts, respectively. Conclusion. Intact endothelial and contractile function of coronary artery may be obtained after 8 hours of non-ischemic heart preservation.
Introduction
The present standard of clinical heart preservation for transplantation consists of flushing the coronary vessels with a cold cardioplegic solution followed by static ischemic storage at 4 °C.[Citation1] Data from the ISHLT registry on 10,473 patients receiving heart transplantations between 2006 and 2011 reveals an increase in hazard ratio for 1-year mortality from 1.0 at 3 hours to 1.9 at 6 hours of cold ischemia.[Citation2] Most heart transplant surgeons therefore try to limit the cold ischemic time to 4 hours.
In a study on porcine hearts, flushing the heart with 1 L of 4 °C St. Thomas Cardioplegic Solution, using a perfusion pressure of 60–65 mmHg, significantly reduced endothelium-dependent relaxation (EDR); flushing followed by 12 hours of cold storage at 4 °C significantly impaired EDR which worsened still more after 24 hours of reperfusion.[Citation3] A correlation was found (p < 0.001) between high coronary vascular resistance and low EDR [Citation3] and the coronary flow and cardiac output were significantly reduced in hearts with low EDR.[Citation4] The endothelium thus plays a critical role in the maintenance of normal vessel function. Endothelial cells produce a variety of substances which control vascular permeability, vessel tone, coagulation, fibrinolysis and inflammatory response. When well preserved, the endothelium is antithrombogenic and yet promotes platelet aggregation and coagulation if injured. Vasospasm, intimal hyperplasia and accelerated atherosclerosis can occur as a result of inadequate endothelial preservation.[Citation5,Citation6]
Recently, a study was published demonstrating safe orthotopic transplantation of porcine hearts harvested 24 hours after brain death and preserved for 24 hours with non-ischemic perfusion before being transplanted.[Citation7] A clinical study with this new method is planned with the intention, as a first step, to demonstrate safe donor heart preservation for 8 hours.
The aim of the present study was to study the EDR and the contractile function of the coronary artery after 8 hours of heart preservation with this new method of non-ischemic perfusion.[Citation7]
Material and methods
Anesthesia and animal preparation
Swedish domestic pigs with a mean weight of 56 kg (range 40–75 kg) were used. All animals received care in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Directive 2010/63/EU) and the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research. The ethics committee of the University of Lund approved the study (No. M389-12).
Anesthesia was induced in the animals using an intramuscular injection of atropine 0.5 mg (Atropine, Mylan AB, Stockholm, Sweden), xylazine 100 mg (Rompun Vet, Bayer, Solna, Sweden), and ketamine 20 mg/kg body weight (Ketaminol Vet. Intervet, Boxmeer, Netherlands). Fentanyl 4 μg/kg body weight (Fentanyl B. Braun, Melsungen, Germany) and midazolam 0.4 mg/kg body weight (Midazolam Panpharma, Panpharma S.A., Trittau, Germany) were given intravenously through an ear vein catheter before tracheostomy. Anesthesia and muscle relaxation were maintained with a continuous intravenous infusion of ketamine (10 mg/kg/h) and xylazine (1.5 mg/kg/h).
Volume-controlled and pressure-regulated ventilation was used, with a minute volume of 100–150 ml/kg body weight and a frequency of 20 breaths/minute. Positive end-expiratory pressure was adjusted to 5 cm H2O and the inspired oxygen fraction was 0.5. End-tidal CO2 was maintained between 4.8 and 5.3 kPa by regulating minute volume.
Aortic pressure and central venous pressure were measured with catheters placed into ascending aorta and superior vena cava, respectively. Body temperature was measured with a temperature probe in the trachea. A median sternotomy was performed and the heart was exposed. Heparin 500 IU/kg body weight (Heparin LEO, City, Company) was given intravenously.
Design of the study
The animals were divided into three groups: fresh controls, intermittent perfusion (I), and continuous perfusion (C). Segments of the coronary artery were investigated in organ baths, immediately for fresh controls, and after 8 hours of preservation for the other two groups.
Fresh controls
Ventricular fibrillation was induced with a current pulse from a 9 V battery source applied to the surface of the heart. This was done so that excision of the coronary artery could be immediately performed without a moving heart. The distal part of the left anterior descending coronary artery (LAD) was excised from the heart and placed in a dissection bath with Krebs solution. Residual blood was removed by a gentle flow of Krebs solution through the lumen. Using an operation microscope, the LAD was dissected free from surrounding tissue, cut into ring segments about 3 mm in length, and transferred into organ baths containing Krebs solution (37 °C) bubbled with 95% O2 and 5% CO2.
The heart preservation system
The heart preservation system used has been described in detail elsewhere.[Citation7] The main components of the system are as follows: an automatic pressure and flow-controlled perfusion system, an automatic gas exchange system, a leucocyte filter, an arterial filter, a heater-cooler unit, an autonomous power unit, and a programmable sequencer.
The perfusate consists of an albumin-containing hyperoncotic cardioplegic nutrition solution with hormones and erythrocytes (for composition, see ). A baby-feeding catheter was inserted into the coronary sinus and samples for blood analysis (Radiometer ABL 725, Copenhagen, Denmark) were taken after 2 and 8 hours. Temperature corrections were made for blood gases.
Table 1. The perfusion medium used for 8-hour heart preservation.
Intermittent preservation (I)
The aorta was cannulated with a tube connected to the perfusion system for infusion of the perfusion medium. The hearts were perfused at 8 °C with a perfusion pressure of 20 mmHg in cycles of 15 min perfusion and 60 min without perfusion. The coronary flow was in the range of 100–200 mL/min during the 15-minute perfusion phase. Before each perfusion, the preservation medium was automatically mixed for 2 min and the perfusion lines were recirculated to remove erythrocyte sequestration. Each perfusion cycle included pressure sensor zero calibration. A perfusion pressure of 20 mmHg is enough to supply adequate amount of oxygen and nutrition to a cardioplegic heart at 8 °C.[Citation7]
Continuous preservation (C)
The aorta was cannulated as for I. The hearts were continuously perfused with a pressure of 20 mmHg for 8 hours. The coronary flow was in the range of 120–200 mL/min.
Drugs used in the organ baths
The following were included in the organ baths: substance P, which is a selective drug for inducing EDR in pigs (acetylcholine for this purpose does not work in pigs); papaverine, which is an endothelium independent vasodilator; U46619, which is a thromboxane A2 analogue that gives strong and stable vasocontraction; and 127 mmol/L potassium-Krebs solution, which also gives strong and stable vasocontraction, caused by depolarization of the vascular smooth muscle cells.
Recording of contractility and EDR and endothelium-independent relaxation
Isometric tension was measured in a 5 ml water-mantled Perspex bath containing Krebs solution, bubbled with 95% oxygen and 5% carbon dioxide to give a pH of approximately 7.4 at 37 °C. The composition of the Krebs solution was (in mmol/L) NaCl 119, NaHCO3 18, KCl 4.6, NaH2PO4 1.2, MgCl2 1.2, CaCl2 1.5, and d-glucose 5.5. Each ring segment of the coronary vessel was suspended between two metal holders (0.2 mm in diameter). One holder was attached to a Grass FT03 transducer (Grass Instrument Co, Quincy, MA) connected to a computer armed with specially designed data acquisition software for continuous recording of the isometric tension. The other metal holder was fixed to an adjustable unit that was used to repeatedly stretch the vessel segments to a basal tension between 8 and 10 mN.
Pilot experiments showed that maximum response could be obtained at this tension. Two contractions were induced with 127 mmol/L potassium-Krebs solution. The second contraction was defined as a maximum contraction elicited by complete depolarization of the vessel segment. Pilot experiments showed that the variability between the second and a third potassium-Krebs induced contraction varied less than 10%, therefore the second contraction was used. After washing the bath repeatedly with normal Krebs solution to reach basal tension, a contraction was induced with U46619 (a thromboxane A2 analogue) at a concentration of 3 × 10−8 mol/L. Pilot experiments showed that this concentration gave 50–85% of maximal contraction induced by U46619. When a plateau was reached, increasing concentrations (10−9 to 10−4 mol/L) of Substance P were cumulatively added to the bath. Finally, the endothelium-independent vasodilator Papaverine (10−4 mol/L) was added to the bath to ascertain whether complete relaxation had been obtained. The distance between the metal holders was measured at basal tension using a microscope armed with an ocular scaler. The diameter of the vessel segments was calculated as the distance between the two holders times 2 divided by 3.14.
Measurement of heart water content
Slices with a thickness of 5–10 mm were transversely excised from both ventricles of all the hearts immediately after excision of the coronary vessels. The weight of the slices was measured and recorded as wet weight. An oven (Modell 600, Memmert, Schwabach, Germany) was used to dry the slices at a temperature of 60 °C. Daily weight measurements were made, and when the weight did not decrease after three consecutive measurements, it was recorded as the dry weight. Water content in percent was calculated with the formula: (wet weight – dry weight)/wet weight ×100.
Data analysis
From the concentration–response curves induced by Substance P, the maximum relaxation and the pEC50 values were determined. The pEC50 value was defined as the negative logarithm of the concentration giving half maximal relaxation. Results were expressed as the mean ± standard error of the mean. Student’s t-test for unpaired data was used to compare the difference between the groups. A p-value of less than 0.05 was considered statistically significant.
Results
Before sternotomy, the baseline values for arterial and venous blood pressure and blood gases, heart rate and temperature were normal in all animals.
Size of the coronary artery segments
At basal tension the diameter of the coronary artery segments was 1.51 ± 0.04, 1.51 ± 0.04 and 1.56 ± 0.08 mm for the fresh controls, I and C, respectively. The length of the coronary artery segments was 3.04 ± 0.02 mm. There were no statistically significant differences between the three groups.
Blood gases in coronary sinus
The blood gases after 2 and 8 hours of preservation are given in . There were no statistically significant differences at 2 hours or at 8 hours of preservation in the either group.
Table 2. Blood gases from coronary sinus during preservation.
Vasocontraction
The contractions induced by potassium-Krebs and U46619 are given in . There were no statistically significant differences between the three groups.
Table 3. Coronary smooth muscle contraction and EDR and endothelium-independent relaxation.
EDR and endothelium-independent relaxation
The concentration–response curves for Substance P are shown in . The maximal relaxation of the contraction induced by 3 × 10−8 mmol/L U46619 was 93%, 96% and 96% for the fresh controls, I and C, respectively (). The corresponding pEC50 values were 6.21 ± 0.11, 6.31 ± 0.06 and.44 ± 0.07. There were no statistically significant differences between the three groups.
Figure 1. EDR elicited by Substance P on a thromboxane A2-induced stable contraction. Results are given as mean ± SEM, n = 8.
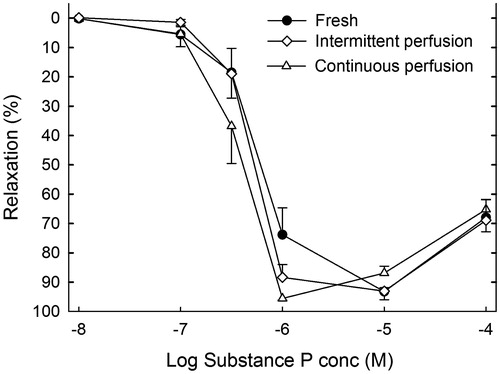
The relaxation elicited by 10−4 M papaverine was 104.2 ± 0.3%, 103.3 ± 0.7% and 101.7 ± 0.2% for the fresh controls, I and C, respectively (). There were no statistically significant differences between the three groups.
Water content of the myocardium
Neither continuous nor intermittent perfusion caused tissue edema in the heart. The water content of the myocardium for the fresh controls, and for I and C after 8 hours of preservation was 79.5 ± 0.2%, 79.0 ± 0.4% and 79.0 ± 0.3%, respectively (ns).
Discussion
For adequate non-ischemic perfusion preservation of a cardioplegic heart, several factors need to be considered: the composition and temperature of the perfusate; the oncotic pressure in the perfusate, which must be higher than the perfusion pressure used to prevent edema; the gas exchange capacity of the perfusate, which must provide an adequate supply of oxygen to prevent hypoxia; and the buffer capacity, which must keep the pH within physiological limits throughout the preservation time. The heart preservation system used in the present study is the same as that used in the study demonstrating safe heart preservation for 24 hours;[Citation7] for a detailed description of this heart preservation system and a discussion of the above mentioned factors, see Steen et al. [Citation7] In that study, the preservation was done in cycles of 15 minutes perfusion and 60 minutes none perfusion. In the present study, we also added a group with continuous flow to investigate if that would affect the endothelial function differently from intermittent perfusion. The preservation was excellent in both groups and did not differ from fresh controls.
Hypothermia improves ischemic tolerance by reducing the metabolism. It has been taken for granted that organs can tolerate static ischemic storage at 4 °C for at least a few hours without impairment of function. However, the pioneers in modern clinical lung transplantation, Cooper, Patterson, Keshavjee and coworkers, have published an extensive study on the effect of ischemic time and temperature on lung preservation.[Citation8] They found that preservation of lungs at 10 °C is superior to preservation at 4 °C or at 15 °C. This was an “unanticipated finding that at 10 °C lung function is much better preserved for a given period of time than at 4 °C, the preservation temperature commonly used clinically for all transplanted organs”.[Citation8] Nakamoto and coworkers have also published a study on the optimal temperature for static ischemic preservation of the pulmonary circulation.[Citation9] They used indocyanine green dilution rate of effluent and histological distribution of carbon particles and found the optimal temperature to be between 8° and 9 °C. The grafts preserved at 8 °C showed intact microvasculature even in histological investigation, whereas those preserved at 4 °C showed vascular obstruction with accompanying prominent perivascular cuffing. The authors suggest that “the most common hypothermic ischemic injury during preservation by topical cooling is pulmonary vascular obstruction, which might be induced at temperatures lower than the critical temperature of 6° to 7 °C”.[Citation9] The heart is an organ extremely rich in capillaries, which may react to cooling in a similar way as the lung.[Citation8–9] With non-ischemic preservation, temperatures higher than 8 °C can be used, even normothermia. To have a safety margin in case a technical problem should occur with the perfusion, we decided to preserve the hearts at 8 °C, taking into consideration the above-mentioned studies indicating that temperatures lower than this may not be optimal.[Citation8–9] Furthermore, 8 °C was the temperature used in the study demonstrating safe non-ischemic heart preservation for 24 hours using cycles of 15 minutes perfusion and 60 minutes non-perfusion.[Citation7]
Disturbance of the functional integrity of the vascular endothelium, known as “endothelium dysfunction”, plays a significant role in myocardial ischemia-reperfusion injury.[Citation3–6,Citation10–12] The vascular endothelium is a single layer of cells that lines the entire circulatory system. A well-preserved endothelium counteracts leucocyte adhesion and platelet aggregation, and actively regulates vascular tone by producing vasoactive substances. For safe prolonged preservation of donor hearts for transplantation, an adequate preservation of the endothelium is vital, and cardiac allograft vasculopathy continues to limit the long-term success of cardiac transplantation. Vascular lesions are the result of cumulative endothelial injuries, including ischemia-reperfusion injury and alloimmune responses, which are worsened if the endothelium is not optimally preserved from the beginning.[Citation13] Cardiac allograft vasculopathy and malignancy are the most important causes of death in patients who survive the first year after transplantation.[Citation14]
The conclusion of the present study is that the endothelial and contractile function of the coronary artery can be fully preserved for 8 hours with non-ischemic perfusion-preservation.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study was supported by a grant from Hans-Gabriel and Alice Trolle-Wachtmeister’s Foundation for Medical Research.
References
- Minasian SM, Galagudza MM, Dmitriev YV, et al. Preservation of the donor heart: from basic science to clinical studies. Interact Cardiovasc Thorac Surg. 2015;20:510–519.
- ISHLT. (International Thoracic Transplant Registry Steering Committee). Adult Heart Transplantation Statistics; 2013. Available from: https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry.
- Budrikis A, Liao Q, Bolys R, et al. Effects of cardioplegic flushing, storage, and reperfusion on coronary circulation in the pig. Ann Thorac Surg. 1999;67:1345–1349.
- Budrikis A, Bolys R, Liao Q, et al. Function of adult pig hearts after 2 and 12 hours of cold cardioplegic preservation. Ann Thorac Surg. 1998;66:73–78.
- Steen S. Preservation of the endothelium in cardiovascular surgery-some practical suggestions-a review. Scand Cardiovasc J. 2001;35:297–301.
- Yang Q, He G-W, Underwood MJ, et al. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection. Am J Transp Res. 2016;8:765–777.
- Steen S, Paskevicius A, Liao Q, et al. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J. 2016;50:193–200.
- Wang L-S, Yoshikawa K, Miyoshi S, et al. The effect of ischemic time and temperature on lung preservation in a simple ex vivo rabbit model used for functional assessment. J Thorac Cardiovasc Surg. 1989;98:333–342.
- Nakamoto K, Maeda M, Taniguchi K, et al. A study on optimal temperature for isolated lung preservation. Ann Thorac Surg. 1992;53:101–108.
- Shimokawa H, Yasuda S. Myocardial ischemia: current concepts and future perspectives. J Cardiol. 2008;52:67–78.
- Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601.
- Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:3–10.
- Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–2141.
- Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult heart transplantation report: 2006. J Heart Lung Transplant. 2006;25:869–879.
