Abstract
In remote ischemic preconditioning (RIPC) short periods of non-lethal ischemia followed by reperfusion of tissue or organ prepare remote tissue or organ to resist a subsequent more severe ischemia-reperfusion injury. The signaling mechanism of RIPC can be humoral communication, neuronal stimulation, systemic modification of circulating immune cells, and activation of hypoxia inducible genes. Despite promising evidence from experimental studies, the clinical effects of RIPC have been controversial. Heterogeneity of inclusion and exclusion criteria and confounding factors such as comedication, anesthesia, comorbidities, and other risk factors may have influenced the efficacy of RIPC. Although the cardioprotective pathways of RIPC are more widely studied, there is also evidence of benefits in CNS, kidney and liver protection. Future research should explore the potential of RIPC, not only in cardiac protection, but also in patients with threatening ischemia of the brain, organ transplantation of the heart, liver and kidney and extensive cardiovascular surgery. RIPC is generally well-tolerated, safe, effective, and easily feasible. It has a great prospect for ischemic protection of the heart and other organs.
Introduction
Ischemic preconditioning (IPC) is a phenomenon in which short periods of non-lethal ischemia protect against a subsequent and more severe ischemia-reperfusion insult. Local ischemic preconditioning was discovered in the mid-1980s in experimental models of myocardial infarction. A significant reduction in the size of an infarction was observed when four 5-minute cycles of coronary occlusion were executed prior to 40 minutes of coronary occlusion in a canine model.[Citation1] A few years later, animal studies showed that ischemia in one coronary artery territory protects myocardium perfused by another coronary artery.[Citation2] The finding that ischemia in a non-cardiac organ protects the heart at a distance led to the concept of remote ischemic preconditioning (RIPC).[Citation3] In 2002 the first clinically relevant large animal (porcine) model showed that RIPC performed by cycles of hind limb tourniquet occlusions reduced the size of myocardial infarction. This finding aroused interest to subsequent clinical trials in humans.[Citation4]
Pathways from remote organ to target protection
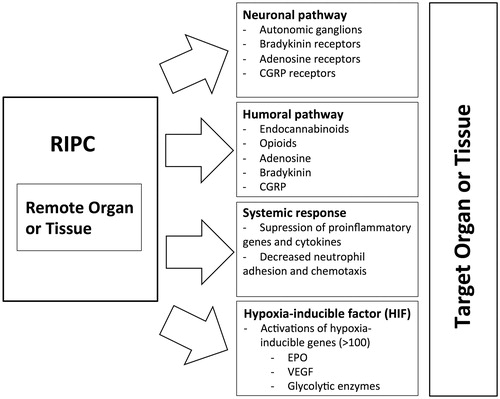
The most fascinating part of RIPC is its ability to produce protective factors from distance to the actual target tissue or organ. Several signaling mechanisms of RIPC have been proposed, including humoral pathways, neuronal stimulation, communication via systemic modification of circulating immune cells, and activation of hypoxia inducible genes ().
Humoral pathway
It has been suggested that humoral factors generated in remote preconditioning organs have to be washed out into the bloodstream toward the more susceptible organ (e.g. heart, central nervous system (CNS)). This was shown in a study where cardioprotection was induced in an unpreconditioned rabbit heart using the transfer of coronary perfusate from a RIPC heart. This protective effect was also detected while studying cross-species transfer.[Citation5] The presence of a circulating cardioprotective factor after RIPC was also demonstrated in a porcine model with a denervated donor heart.[Citation6]
Several studies have attempted to identify a specific circulating factor that might be the transmitter of RIPC. The shear-stress-related release of nitric oxide with reactive hyperemia after transient limb ischemia is expected to increase cardioprotective nitrite levels in the blood.[Citation7] Plasma levels of cardioprotective stromal-derived factor-1α were increased after RIPC in rats.[Citation8] A microribonucleic acid (microRNA) was shown to play a role in the pre-conditioning effect of RIPC.[Citation9] Extracellular vesicles (exosomes and microvesicles) are membrane-bound structures secreted by mammalian cells and these vesicles were showed to participate in a carrier mechanism of the cardioprotective effect of RIPC.[Citation10] Although aforementioned factors are clearly humoral transfer signals, they cannot entirely explain RIPC phenomenon.
As ischemia is an unspecific injury that results in disturbances in multiple cellular processes, an obvious explanation is that RIPC activates the release of several humoral factors and involves multiple endogen-protective mechanisms. However, the preconditioning stimulus has to be recognized by the cellular sensor, and the cell must be prepared for the upcoming stress. The elements of RIPC might include the sensor of the stress signal, transducers of the stimulus, or effectors of the tolerance.[Citation11]
Neuronal pathway
Evidence from several experimental models proposes that transient ischemia in itself is not a requirement for remote protection. Multiple alternative triggers capable to result in ischemia protection in remote organ have been proposed. Peripheral nociception, initiated by surgical skin incision, “remote pre-conditioning of trauma”, have been demonstrated to induce remote cardioprotection.[Citation12] Local nerve stimulation appears to have an effect similar to RIPC, whereas a nerve blocker of the peripheral nerve abolishes the protection.[Citation13] Cardioprotection by RIPC seems to be dependent on afferent innervation of the remote organ and intact parasympathetic activity, while delayed remote postconditioning relies on different signaling pathways.[Citation14] The recent study in rat model have shown that the circulating factors of RIPC are produced and released into the systemic circulation by the visceral organs innervated by the posterior gastric branch of the vagus nerve.[Citation15] In brain protection, neuronal involvement in RIPC has been reported in several studies, mainly via a blockade of sensory inputs that reduced the beneficial effects of RIPC.[Citation16,Citation17] In contrast, evidence for spinal cord protection and neuronal pathway involvement is not as strong, as the effects of RIPC on the spine were not eliminated by a ganglionic blocker, but by antioxidants, indicating the importance of humoral factor.[Citation18]
The neuronal pathway includes the somatosensory and autonomous nervous systems and the spinal cord. The causal involvement of peripheral nociceptive sensory nerves is obvious, but the nature and transfer of the released transmitter molecule to the target organ through humoral or neuronal pathways remains unclear. It has been proposed that endogenous substances (adenosine, bradykinin, calcitonin gene-related peptide (CGRP)) are released in a remote preconditioned organ and further stimulate afferent nerve fibers, which transmit the effects to the target organ, promoting protective cellular processes.[Citation19] However, ischemic protection was able to transfer to a recipient heart by blood-derived dialysate after local peripheral adenosine or capsaicin administration or RIPC, suggesting humoral transfer of a neuronally released signal molecule.[Citation13] Human study showed that the diabetic neuropathy was associated with impaired response to the dialysate mediated remote ischemic conditioning indicating that the release mechanism involves neuronal pathways.[Citation20]
When knowledge of mediating factors accumulates, it is evident that the protective cascade is a complex process including both humoral and neuronal pathways. More information is needed on the effects on different organs because it has not been fully confirmed that the same factors that affect the CNS also affect (for example) the heart.
Systemic response
Evidence of suppression of the inflammatory response is detected after RIPC. It has been shown that specific adhesion molecules (intercellular adhesion molecule-1 (ICAM-1), P-selectin) decrease in cell membranes after RIPC.[Citation21] It seems that leukocyte–endothelium interactions are regulated by RIPC, and a decreased number of activated leukocytes is detected in the ischemic area.[Citation22] Moreover, RIPC seems to reduce pro-inflammatory gene expression in circulating leukocytes. These genes are associated with leukocyte activation, cell adhesion, and intracellular signaling.[Citation23] These studies suggest that RIPC has a direct effect on the inflammatory system, and thus prevents exacerbation of ischemic injuries.
Molecular mechanisms of RIPC
Mitochondrial dysfunction is thought to be the major contributor to ischemia–reperfusion-mediated injury. Thus, it seems logical that several reviews of the mechanisms underlying RIPC and IPC point to evidence that several protein kinases are activated during ischemic preconditioning and are targeted toward maintenance of mitochondrial function, especially inhibition of the opening of the mitochondrial permeability transition pores (mPTP).[Citation11,Citation24]
Initially, sublethal ischemic insults lead to transient decreases in cellular ATP levels. It has been demonstrated that the ATP/ADP ratio decreases after minutes of mitochondrial ATP synthase inhibition.[Citation25] The consequential increase in ATP metabolites, such as ADP, AMP, and adenosine, may act to induce ischemia tolerance pathways. For example, adenosine pretreatment has been found to attenuate ischemic insults in in vivo and in vitro studies.[Citation26,Citation27] The decreased ATP/ADP ratio may also directly or indirectly activate mitochondrial K+ATP channels. These channels seem to play a crucial role in ischemic tolerance.[Citation26]
The studies to identify the signaling molecules or mechanisms of RIPC have mostly focused on signals identified in local ischemic precondition studies.[Citation28] However, most signaling mechanisms have thus far only been determined in rodent hearts, and further studies are warranted to translate findings to larger mammals or humans. With these limitations, there is evidence for a causal involvement of the adenosine,[Citation29] bradykinin,[Citation30] interleukin-10 (in delayed RIPC),[Citation31] and stromal-derived factor-1α.[Citation8]
One of the key elements of ischemic tolerance is the adenosine triphosphate-sensitive potassium channel (KATP channel), located in the sarcolemma and mitochondria. The opening of KATP channels is considered to be one of the main mechanisms of RIPC. In animal studies, the adenosine A1 receptor antagonist weakened the protective effects of RIPC.[Citation32] The KATP channel activator diazoxide mimicked the protective effect of preconditioning, also implying that the KATP channel is the mediator of the protective effect.[Citation33]
Bradykinin B2 receptor activation results in protein kinase PKCɛ activation.[Citation30] The PKCɛ translocation to mitochondria accelerates the activity of mitochondrial KATP channels.[Citation34] The action of interleukin 10 results in increased phosphorylation of protein kinase B and endothelial nitric oxide synthase.[Citation31]
The hypoxia-inducible transcription factor (HIF), consisting of a labile α subunit and a stable β subunit, accumulates during hypoxia. Hypoxia response is triggered by the HIF through the induction of over 100 genes whose products promote cellular survival under ischemic conditions, such as those for erythropoietin (EPO), transferrin, vascular endothelial growth factor (VEGF), and many glycolytic enzymes. The amount of HIF accumulation depends on the alpha subunit. The HIF α subunit is regulated through prolyl hydroxylation by α-ketoglutarate (αKG)-dependent dioxygenases known as EGLNs. Of the three EGLN paralogs, EGLN1 is the primary regulator of HIFα. EGLNs act as “O2 sensors” in metazoans and coordinate cellular responses that promote adaptation to hypoxia and ischemia.[Citation35]
RIPC is known to increase HIF-1-α protein levels in cardiac atrial tissue in humans.[Citation36] Both local and remote preconditioning is attenuated in HIF1α± mice.[Citation37] However, RIPC was demonstrated to induce cardioprotection in partially HIF-1-α-deficient and pharmacologically HIF-1-α-inhibited mice and rats, indicating the complexity of mechanisms behind the protective effects of RIPC.[Citation38] These results support that HIF protects the heart during acute myocardial infarct. However, long-term changes in HIF levels might cause undesirable effects such as decreased mitochondrial function, which would be an unfortunate side effect in any therapies attempting to protect the myocardium via HIF modulation.[Citation39]
Timing of remote ischemic preconditioning
In most studies the RIPC is executed 30–40 minutes prior to subsequent ischemic insult. But pre-treatment is not essential for RIPC-induced protection. Reduction of the infarction size has also been shown with simultaneous application of the RIPC during coronary occlusion (remote ischemic per-conditioning) or at the time of reperfusion (remote ischemic post-conditioning).[Citation40] However, there are two temporally distinct types of RIPC: early and delayed protection. Both types of ischemic tolerance have been detected in the brain and the heart, although the brain usually appears to follow the delayed pattern. It is widely accepted that the early type of protection is independent of protein synthesis, primarily targeting posttranslational modifications, and the protective effect is brief and transient (lasting minutes), whereas the delayed pattern involves new protein synthesis and lasts hours to days.[Citation41]
RIPC and myocardial protection
The effects of RIPC on cardiac protection have been widely studied. Despite promising results in animal models, the clinical effects of RIPC are controversial. The first small clinical study of RIPC in coronary artery bypass graft surgery (CABG) patients could not demonstrate differences in creatine kinase myocardial band.[Citation42] However, a study of RIPC in pediatric patients who underwent surgical repair of congenital heart defects showed reduced cardiac enzyme release.[Citation43] The most of earlier and often small clinical studies showed attenuated biomarker release demonstrating cardioprotective potential of RIPC. This was first associated with beneficial clinical outcome in a single-center study evaluating the effect of RIPC on patients undergoing CABG. RIPC provided perioperative myocardial protection and significantly improved the prognosis of patients.[Citation44] After percutaneous coronary intervention (PCI), major adverse cardiac and cerebral events were significantly lower in RIPC-treated patients.[Citation45] RIPC before hospital admission in conjunction with PCI increased myocardial salvage in a randomized trial [Citation46] and remote ischemic post-conditioning with PCI reduced infarct size.[Citation47] The combined RIPC and postconditiong along with PCI in ST-segment elevation myocardial infarction (STEMI) improved myocardial salvage.[Citation48] RIPC prior to primary PCI in STEMI reduced myocardial infarction size in randomized trial.[Citation49] However, a large systematic meta-analysis of 2200 patients undergoing cardiovascular surgery did not reveal significant benefits regarding perioperative adverse events.[Citation50] Heterogeneity of inclusion and exclusion criteria and the type of preconditioning stimulus might limit the evaluation of RIPC effects. Confounding factors such as age, comedication, anesthesia, comorbidities, and other risk factors may have influenced the efficacy of RIPC.[Citation51]
A recently published clinical multicenter, double-blinded, randomized, prospective, controlled human trial called the RIPHeart study, with patients undergoing CABG with or without valve surgery using cardiopulmonary bypass, could not demonstrate significant differences between the treatment groups.[Citation52] Similar results were published in the ERICCA study with patients undergoing CABG with or without valve surgery.[Citation53] Though the ERICCA and the RIPHeart studies are to be commended for excellent study protocol and execution, there are some important points that one should consider before rejecting the notion of RIPC altogether. A propofol anesthesia was used on all the patients, which has the effect of dampening RIPC-induced cardioprotection.[Citation54] Concomitant therapy with beta-blockers [Citation55] and statins [Citation56] is cardioprotective and may interfere with the cardioprotective effect of RIPC. The limitation was also that the patients were quite heavily laden with co-morbidities to show any benefits of RIPC. The ERICCA and the RIPHeart studies have shown us that RIPC is not a cure-all protection method that benefits all patient groups that undergo cardiac surgery with a cardiopulmonary bypass. These results are a significant milestone in assessing the efficacy and effects of RIPC in intermediate risk populations, but there is still justification to study the possible effects of RIPC in smaller subgroups to ascertain whether it could be beneficial to a specific patient group. Remote ischemic preconditioning might prove beneficial, for example, in healthier patients undergoing a specific type of operation.
RIPC and cerebral protection
Although the cardioprotective pathways of RIPC are more widely studied, there is also evidence of benefits in CNS protection. Neuronal ischemic preconditioning was demonstrated in vivo 1990.[Citation57] Remote ischemic protection of the brain was first tested in a rat model of global cerebral ischemia, and the results were promising.[Citation58] Experimental and clinical studies about the neuroprotective effect of RIPC on the CNS have been rather limited so far. In a large experimental animal model with 1 hour of HCA, significantly better EEG recovery rates, superior behavioral scores, and better histopathological scores were recorded in the RIPC arm of the study 7 days post-operation.[Citation59] In subsequent studies RIPC reduced the amount of circulating cerebrocortical leukocytes and provided a degree of cell and organ preservation.[Citation22]
Adults undergoing carotid endarterectomy benefitted from RIPC.[Citation60] More interestingly, RIPC has been associated with significantly better motor learning in healthy adults.[Citation61] Additionally, RIPC has shown promising results in focal brain ischemia as an adjunct to thrombolysis in acute ischemic strokes, and was able to reduce the risk of infarction in brain tissue.[Citation62] However, the main brain structure mediating this protection has not been identified yet, indicating that the studies on this subject are justified.[Citation63]
RIPC and spinal cord protection
Despite the favorable trend in the outcome of extensive thoracoabdominal aneurysm repair, the incidence of temporary or permanent spinal cord injury still varies between 3% and 20% depending on the extent of the aortic pathology and operative urgency.[Citation64] Nevertheless, paraplegia and paraparesis still exist postoperatively, leaving room for new protective innovations. The beneficial effect of RIPC has been documented in the experimental studies of spinal cord injury.[Citation65] A recent study documented a significant increase in MEP amplitude and a decrease in onset latency after hind limb RIPC in a porcine model.[Citation66] Spinal cord protection by RIPC was confirmed in a study of 40 patients who underwent cervical decompression surgery. A significantly better recovery rate was observed in RIPC-treated patients 3 months after surgery.[Citation67] Additionally, the cardioprotective effect of RIPC was lost if the spinal cord was chemically blocked.[Citation68] In contrast, the effect of RIPC on the spinal cord was not abolished by ganglionic blockers but by using antioxidants.[Citation18] Thus, the spinal cord might play a role in transmitting the effect of ischemic bouts from the periphery to the target organ, but the protective mechanisms might be different in various organs. Remote ischemic preconditioning causes initial oxidative stress, which seems to irritate the spinal cord either via neural, humoral, or systemic (immunological) pathways.
Although no clinical trials have been conducted to date, RIPC has the potential to become a novel therapeutic modality for the amelioration of ischemic insults to the spinal cord in the setting of thoracoabdominal aortic aneurysm repair.
Safety of remote ischemic preconditioning
Remote ischemic preconditioning is beneficial in many ways, and it is smoothly applicable to cardiac operations as well as to aortic arch and descending aortic aneurysm operations, using both endovascular and open procedures. It is not surprising that, in clinical studies of RIPC, arm or leg ischemia is most often used because of the ease of application. RIPC is generally well-tolerated and safe. The microdialysis study measuring possible injurious effects of RIPC on the limbs did not find any indications of permanent cell damage.[Citation69] The clinical trial in phase I tested the safety and feasibility of RIPC and did not find any indications of neurovascular injury. The only disadvantage was the temporary pain created by inflation–deflation cycles.[Citation70]
Conclusions
Numerous positive findings from experimental animal works and human studies support the protective effect of RIPC for ischemia-reperfusion injury of the heart and other organs.[Citation71] Details of local release of the protective signal at the remote site and participation of neuronal and humoral pathways are not clear yet. Also, signal transfer to the target organ is partly indefinite. Translation of RIPC strategies from successful animal experiments to the clinic has been somewhat disappointing. Findings from early experiments were neither unequivocal nor confirmed in larger mammalian models and planning of clinical trials was incomplete.[Citation51] Upcoming studies should concentrate on better understanding of the signal transduction to solve the confounding influence of comorbidities and comedication. RIPC distinctly has a feasible prospect for ischemic protection of the heart and other organs. Future research should explore the potential benefit of RIPC, not only in cardiac protection, but also in patients with threatening ischemia of the brain, organ transplantation of the heart, liver and kidney, and extensive cardiovascular surgery.[Citation72]
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136.
- Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic “ustained coronary occlusion” protects remote virgin myocardium from subsequent sustained coronary. Circulation. 1993;87:893–899.
- Gho BC, Schoemaker RG, van den Doel MA, et al. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200.
- Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883.
- Dickson EW, Lorbar M, Porcaro WA, et al. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–H2457.
- Konstantinov IE, Li J, Cheung MM, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695.
- Hepponstall M, Ignjativic V, Binos S, et al. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. Plos One. 2012;7:e48284.
- Davidson SM, Selvaraj P, He D, et al. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res Cardiol. 2013; 108:377.
- Li J, Rohailla S, Gelber N, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423.
- Giritz Z, Varga ZV, Baranyai T, et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78.
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448.
- Jones WK, Fan GC, Liao S, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9.
- Redington KL, Disenhouse T, Strantzas SC, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241.
- Basalay M, Barsukevich V, Mastitskaya S, et al. Remote ischaemic pre- and delayed postconditioning - similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012;97:908–917.
- Mastitskaya S, Basalay M, Hosford PS, et al. Identifying the source of a humoral factor of remote (Pre)conditioning cardioprotection. PLoS One. 2016;11:e0150108.
- Malhotra S, Naggar I, Stewart M, et al. Neurogenic pathway mediated remote ischemic preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011;1386:184–190.
- Wei D, Ren C, Chen X, et al. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7:e30892.
- Dong HL, Zhang Y, Su BX, et al. Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology. 2010;112:881–891.
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386.
- Jensen RV, Stottrup NB, Kristiansen SB, et al. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107:285.
- Peralta C, Fernandez L, Panes J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–113.
- Yannopuolos FS, Arvola O, Haapanen H, et al. Leg ischaemia before circulatory arrest alters brain leukocyte count and respiratory chain redox state. Interact Cardiovascul Thorac Surg. 2014;18:272–277.
- Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150.
- Thompson JW, Narayanan SV, Koronowski KB, et al. Signaling pathways leading to ischemic mitochondrial neuroprotection. J Bioenerg Biomembr. 2015;47:101–110.
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996;66:403–411.
- Blondeau N, Plamondon H, Richelme C, et al. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–474.
- Hiraide T, Katsura K, Muramatsu H, et al. Y, Adenosine receptor antagonists cancelled the ischemic tolerance phenomenon in gerbil. Brain Res. 2001;910:94–98.
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175.
- Leung CH, Wang L, Nielsen JM, et al. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drug Ther. 2014;1:7–17.
- Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–H1576.
- Cai ZP, Parajuli N, Zheng X, et al. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol. 2012;107:277.
- Yoshida M, Nakakimura K, Cui YJ, et al. Adenosine A (1) receptor antagonist and mitochondrial ATP-sensitive potassium channel blocker attenuate the tolerance to focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:771–779.
- Kristiansen SB, Henning O, Kharbanda RK, et al. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2005;288:H1252–H1256.
- Wang Y, Takashi E, Xu M, et al. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001;104:85–90.
- Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–417.
- Albrecht M, Zitta K, Bein B, et al. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314.
- Cai Z, Zhong H, Bosch-Marce M, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–470.
- Kalakech H, Tamareille S, Pons S, et al. Role of hypoxia inducible factor-1α in remote limb ischemic preconditioning. J Mol Cell Cardiol. 2013;65:98–104.
- Huang M, Chan DA, Jia F, et al. Short hairpin RNA interference therapy for ischemic heart disease. Circulation. 2008;118:S226–S233.
- Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011;16:260–266.
- Bhuiyan MI, Kim YJ. Mechanisms and prospects of ischemic tolerance induced by cerebral preconditioning. Int Neurourol J. 2010;14:203–212.
- Günaydin B, Cakici I, Soncul H, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–496.
- Cheung MMH, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282.
- Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604.
- Davies WR, Brown AJ, Watson W, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251.
- Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angiplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734.
- Crimi G, Pica S, Raineri C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction. A randomized controlled study. JACC Cardiovasc Interv. 2013;6:1055–1063.
- Eitel I, Stiermaier T, Rommel KP, et al. Cardioprotection by combined intrahospital remote ischaemic preconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J. 2015;36:3049–3057.
- White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188.
- Remote Preconditioning Trialists' Group, Healy DA, Khan WA, et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176:20–31.
- Ferdinandy P, Hausenloy DJ, Heusch G, et al. Interaction of risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174.
- Meybohm P, Bein B, RIPHeart StudyCollaboration, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407.
- Hausenloy DJ, Candillo L, ERICCA Trial Investigators, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417.
- Zaugg M, Lucchinetti E. Remote ischemic preconditioning in cardiac surgery-ineffective and risky? N Engl J Med. 2015;373:1470–1472.
- Suematsu Y, Anttila V, Takamoto S, et al. Cardioprotection afforded by ischemic preconditioning interferes with chronic beta-blocker treatment. Scandinavian Cardiovasc J. 2004;38:293–299.
- Kocsis GF, Pipis J, Fekete V, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;47:1716–1721.
- Kitagawa K, Matsumoto M, Tagaya M, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–24.
- Schurr A, Reid KH, Tseng MT, et al. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–248.
- Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721.
- Walsh SR, Nouraei SA, Tang TY, et al. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg. 2010;44:434–439.
- Cherry-Allen KM, Gidday JM, Lee JM, et al. CE, Remote limb ischemic conditioning enhances motor learning in healthy humans. J Neurophysiol. 2015; 113:3708–3719.
- Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic preconditioning as an adjunct therapy to thrombolysis in patients with an acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167.
- Meller R, Simon RP. A critical review of mechanisms regulating remote preconditioning-induced brain protection. J Appl Physiol. 2015;119:1135–1142.
- Murana G, Castrovinci S, Kloppenburg G, et al. Open thoracoabdominal aortic aneurysm repair in the modern era: results from a 20-year single-centre experience. Eur J Cardiothorac Surg. 2015;49:1374–1381.
- Sapmaz A, Ulus AT, Turan NN, et al. Which type of conditioning method protects the spinal cord from the ischemia-reperfusion injury in 24 hours? Vascular. 2015;23:614–621.
- Haapanen H, Herajärvi J, Arvola O, et al. Remote ischemic preconditioning protects the spinal cord against ischemic insult: an experimental study in a porcine model. J Thorac Cardiovasc Surg. 2016;151:777–785.
- Hu S, Dong HL, Li YZ, et al. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010;22:46–52.
- Wong GT, Lu Y, Mei B, et al. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci. 2012;91:860–865.
- Bilgin-Freiert A, Dusick JR, Stein NR, et al. Muscle microdialysis to confirm sublethal ischemia in the induction of remote ischemic preconditioning. Transl Stroke Res. 2012;3:266–272.
- Gonzalez NR, Connolly M, Dusick JR, et al. Phase I clinical trial for the feasibility and safety of remote ischemic conditioning for aneurysmal subarachnoid hemorrhage. Neurosurgery. 2014;75:590–598.
- Candillo L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown). 2013;14:193–205.
- Heusch G. Remote conditioning: the future of cardioprotection? J Cardiovasc Med (Hagerstown). 2013;14:176–179.