Abstract
Objectives. To study pre- and postoperative atrial fibrillation and its long-term effects in a cohort of aortocoronary bypass surgery patients. Design. Altogether 615 patients undergoing aortocoronary bypass graft surgery in 1999–2000 were studied. Forty-four (7%) had preoperative atrial fibrillation. Postoperative atrial fibrillation occurred in 165/615 patients (27%) while 406/615 patients (66%) had no atrial fibrillation. After a median follow-up of 15 years, symptoms and medication in survivors were recorded, and cause of death in the deceased was obtained. Results. Death due to cerebral ischaemia was most common in the pre- and postoperative atrial fibrillation groups (7% and 5%, respectively, v. 2% among those without atrial fibrillation, p = .038), as were death due to heart failure (18% and 14%, v. 7%, p = .007) and sudden death (9% and 5%, v. 2%, p = .029). The presence of pre- or postoperative atrial fibrillation was an independent risk factor for late mortality (hazard ratios 1.47 (1.02–2.12) and 1.28 (1.01–1.63), respectively). Conclusions. Patients with pre- or postoperative atrial fibrillation undergoing aortocoronary bypass surgery have increased long-term mortality and risk of cerebral ischemic and cardiovascular death compared with patients in sinus rhythm.
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia among patients undergoing aortocoronary bypass graft (CABG) surgery. Up to 9% of the patients present with preoperative AF accompanying their coronary artery disease,[Citation1] and approximately one-third of CABG patients with preoperative sinus rhythm sustain an episode of postoperative AF.[Citation2,Citation3]
Atrial fibrillation per se is associated with a doubled mortality risk and a fivefold increased risk of stroke.[Citation4,Citation5] In cardiac surgery patients, preoperative AF is an independent risk factor for short- and long-term mortality.[Citation1,Citation6,Citation7] An episode of postoperative AF is also associated with decreased long-term survival, which is mainly explained by an increased risk of cardiovascular death.[Citation2,Citation8,Citation9] Both pre- and postoperative AF therefore affect late mortality and morbidity in patients undergoing aortocoronary bypass surgery, and there is a need to study the possible pathophysiological mechanisms in these patients.
Anticoagulation reduces mortality and risk of stroke in AF patients.[Citation10] Although most patients with either kind of AF undergoing CABG surgery have an indication for anticoagulation treatment, according to the CHA2DS2-VASc score, previous studies have suggested an underuse of anticoagulation in this group of patients.[Citation11] This may in fact be one explanation for the higher incidence of stroke seen in CABG patients compared with patients undergoing percutaneous coronary intervention (PCI).[Citation12,Citation13] Concomitant AF surgery is an option in patients with preoperative AF, and has been shown to reduce AF burden and improve AF-related symptoms.[Citation14] However, its effect on stroke risk and mortality remains to be proved. In postoperative AF patients, strategies for rhythm or rate control are equally effective in reducing complications and length of stay.[Citation15]
The aim of the present 15-year follow-up study was to evaluate pre- and postoperative AF as risk factors for long-term mortality among the same cohort of CABG patients. Our hypothesis was that the presence of AF in CABG patients is a major risk factor for late cardiovascular morbidity and mortality, regardless of whether the AF occurs preoperatively or postoperatively.
Materials and methods
Patients
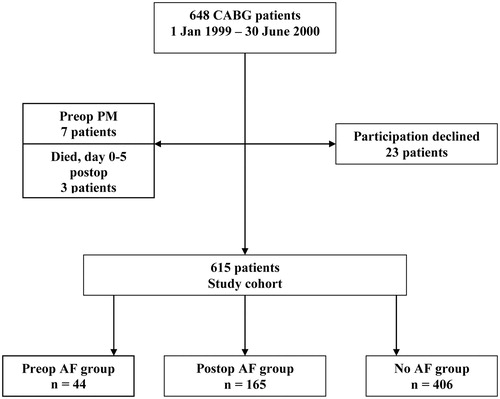
All 648 consecutive patients who underwent primary CABG surgery at the Department of Thoracic and Cardiovascular Surgery, Örebro University Hospital, Örebro, Sweden, between 1 January 1999 and 30 June 2000 were eligible for the study. Patients with preoperative pacemaker (PM) implants (seven patients) and patients not surviving postoperative day 5 (three patients) were excluded. From October 2005 to May 2006, all surviving patients were invited to take part in a follow-up study. Twenty-three patients declined, and the remaining 615 patients formed the study cohort (). The 6-year results from an arrhythmia follow-up of the postoperative AF patients in the cohort have been published elsewhere.[Citation11] No data on the patients with preoperative AF have been published.
From May to June 2013, all surviving patients in the study cohort were again contacted and a follow-up questionnaire was distributed. All patients in the cohort who were deceased as of 31 December 2014 were identified and death certificates collected through the Swedish Nation Cause of Death Register. The patients in the cohort were divided into three groups for comparison: (1) patients with preoperative AF (“Preop AF group”); (2) patients with postoperative AF but no preoperative AF (“Postop AF group”); and (3) patients with no AF (“No AF group”).
The study complies with the Declaration of Helsinki and was approved by the Regional Ethical Committee of Uppsala, Sweden (2004 M-409, amendment 2013-01-23). Signed informed consent was obtained from each patient.
Clinical protocol
After CABG surgery, all patients were monitored by continuous five-lead telemetry (Sirecust 960; Siemens Medical Solutions Diagnostics, Tarrytown, NY) until the second postoperative day. From day 2 until discharge, telemetry was reinstituted in the presence of arrhythmia. A standard twelve-lead electrocardiogram (ECG) was performed on postoperative days 1, 2 and 5, and more often if an arrhythmia was detected. Episodes of arrhythmia were noted on patient surveillance charts, and assessed three times daily and at discharge by the heart surgeon responsible for the case. “Preoperative AF” was defined as AF of any form present in the patient’s history and verified by ECG. Owing to lack of consistent information, further classification of type of AF was not made. “Postoperative AF” was defined as an ECG-verified episode lasting >1 minute during the first 7 postoperative days in patients with no preoperative AF, and the onset and duration of AF were recorded in the patient’s record as well as in the clinical database at the time of discharge. Two independent observers each went through all patients’ records and ECGs twice to collect AF episode data.
The patients with preoperative AF discontinued their warfarin medication 3 days before surgery, and warfarin was resumed on the first postoperative day. Rate control with beta blockers and/or preoperative arrhythmia medication was usually continued postoperatively. The clinical management of postoperative AF at the time of surgery was as follows: preoperative medication including beta blockers and acetylsalicylic acid (ASA) was continued up to the day of surgery, with the exception of warfarin, as discussed above. Unless contraindicated, all patients received beta blocker medication postoperatively. Following the diagnosis of postoperative AF, patients received one or more of the following therapies at the physician’s discretion: a beta blocker, amiodarone, digoxin or verapamil, which were maintained for up to 4 weeks or longer, if tolerated by the patient. Cardioversion was considered if the AF was difficult to rate control. At the end of this period, and coinciding with the first postoperative visit to the outpatient clinic, the medication was discontinued if the rhythm had reverted to sinus. If the patient was still in AF, cardioversion was considered. Patients with AF were given heparin or low-molecular-weight heparin for anticoagulation; warfarin medication was considered if the AF persisted beyond 7 days.
Anaesthetic management was similar in all patients, and included standard monitoring. The CABG was routinely performed with cardiopulmonary bypass using the left internal mammary artery (LIMA) to bypass the left anterior descending artery, and the great saphenous vein to revascularize the circumflex and right coronary artery areas. No arrhythmia surgery procedures were performed in the patients with preoperative AF. After surgery, the patients were transferred to an intensive care unit, extubated after a few hours, and transferred to the patient ward the morning after surgery.
Data management
Patient baseline data, as well as perioperative and postoperative parameters, were prospectively entered into a clinical database. The study database comprised parameters from the clinical database, along with retrospectively collected data from the patient records and laboratory data (missing data <5%). The registered parameters included patient characteristics (age, sex, body mass index (BMI)), concomitant diseases, serum creatinine (μmol/L) obtained the day before surgery, and left ventricular ejection fraction (LVEF) obtained from preoperative echocardiography or angiography. Perioperative and postoperative data included surgical time, length of stay, presence of a postoperative neurological deficit of any kind (defined as a “neurological event”) and medication taken preoperatively and at discharge.
During the period from May to June 2013, all patients in the study cohort were located using the Swedish Population Registry. Surviving patients were contacted and sent a questionnaire including an evaluation of symptoms of angina and irregular heart rhythm, as well as symptoms of stroke and present medication. Based on available data regarding concomitant diseases and age, an estimated CHA2DS2-VASc score was calculated for each patient at the time of follow-up.
All patients in the study who were deceased as of 31 December 2014 were identified through the Swedish National Cause of Death Register. Death certificates were collected and analysed. Primary causes of death were classified into three main groups and eleven subgroups: (1) cardiac: acute myocardial infarction, heart failure, and sudden death; (2) cerebral: cerebral infarction, cerebral haemorrhage, and cerebrovascular insult (due to either bleeding or infarction); and (3) other: malignancy, ruptured aortic aneurysm, infection, miscellaneous causes, and unknown cause of death. In this classification scheme, no information was available regarding the patient’s heart rhythm or previous AF.
Statistics
Data were expressed as means ± standard deviation (SD). Categorical variables were compared using the chi-square test or Fisher’s exact test, while continuous variables were compared using analysis of variance (ANOVA) or the Kruskal–Wallis test for non-parametric distribution. A p-value <.05 was considered statistically significant.
To illustrate the effect of AF on long-term survival, Kaplan-Meier cumulative survival curves were constructed and compared using the log-rank test. Factors determining survival were analysed using Cox proportional hazard analysis. Variables of interest were screened using univariate analysis, and those with a p-value ≤.10 or that were considered to be of clinical importance were entered into a proportional hazard model with no variable selection performed. Statistical analysis was performed using SPSS version 15 (SPSS Inc., Chicago, IL).
Results
Baseline data
In the study cohort, 44/615 patients (7%) had a history of preoperative AF. Postoperative AF occurred in 165/615 patients (27%), while 406/615 patients (66%) had no pre- or postoperative AF (). The baseline data of the three groups are summarized in . The No AF group of patients were younger compared with both other groups, leading to a lower Higgins risk score. The Preop AF patients were more often hypertensive (48%, compared with 36% of Postop AF and 30% of No AF patients) and serum creatinine was elevated compared with the No AF patients. There were no gender differences, and history of coronary or cerebrovascular disease did not differ significantly between the groups.
Table 1. Patient characteristics (n = 615).
Perioperative and postoperative data are summarized in . There were no differences between the groups regarding techniques of revascularization or surgical time. However, Preop and Postop AF patients had an increased length of stay (9.1 days and 9.2 days, respectively), compared with No AF patients (7.4 days). They also had an increased risk of neurological events during hospital stay (7% and 2%, respectively, compared with No AF patients, 1%) and an increased 30-day mortality (2.3% and 2.4%, respectively, compared with the No AF patients, 0.2%).
Table 2. Perioperative and postoperative data.
Cause of death
The median follow-up was 15 years. The total mortality was 36 deaths/44 patients (82%) in the Preop AF group, 105 deaths/165 patients (64%) in the Postop AF group, and 191 deaths/406 patients (47%) in the No AF group ().
Table 3. Causes of death in the study cohort after a median follow-up of 15 years.
Death due to heart failure and sudden death were more common in the Preop and Postop AF groups (heart failure 18% and 14%, v. 7%, p = .007), as was death due to cerebral ischaemia (7% and 5%, v. 2%, p = .038). Death due to myocardial infarction, cerebral bleeding and non-cardiovascular causes did not differ significantly between the groups.
The long-term survival in the three groups is illustrated by the Kaplan–Meier curves in . The results of the Cox regression analysis are summarized in . Age, pre- or postoperative AF, diabetes, and LVEF <30% were univariate predictors of late mortality. In the multivariable analysis, the presence of preoperative or postoperative AF was an independent risk factor for late mortality (hazard ratio (HR) 1.47 (1.02–2.12), p = .038, for preoperative AF; HR 1.28 (1.01–1.63), p = .045, for postoperative AF) after adjusting for differences in age, and for diabetes, hypertension, ejection fraction and gender (). After adjustment for preoperative serum creatinine, the effect of preoperative AF was no longer significant (HR 1.33 (0.92–1.94), p = .132, data not complete).
Figure 2. Kaplan–Meier survival curves for all patients in the study cohort undergoing coronary artery bypass graft (CABG) surgery, 1999–2000. Patients divided into groups with no atrial fibrillation (AF) (No AF), postoperative AF (Postop AF) and preoperative AF (Preop AF). Log-rank test p < .001.
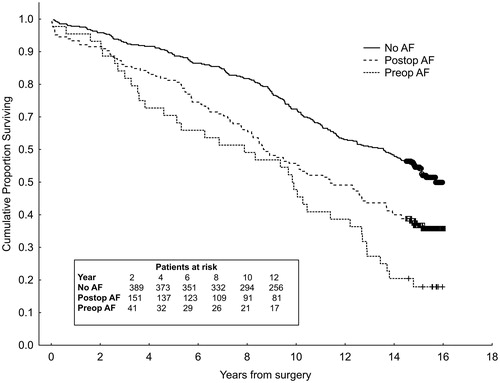
Table 4. Predictors of mortality using cox regression analysis.
Questionnaires
Questionnaires were obtained from 256 out of 326 surviving patients (78%) at 14 years after surgery (). In total, 161/256 patients (63%) were free from angina, with no significant differences between the groups. Subjective symptoms of irregular pulse were most common in the Postop AF group (61%), and hospitalization due to stroke was more common in the Preop AF and Postop AF groups (45% and 15%, respectively, p = .001).
Table 5. Questionnaire results 14 years after aortocoronary bypass graft (CABG) surgery.
Anticoagulation and CHA2DS2-VASc score
Preoperatively, warfarin anticoagulation was administered to 18% of Preop AF patients and 1% of patients in both other groups. At discharge, 26% of Preop AF patients, compared with 4% of the Postop AF patients and 1% of the No AF patients, received warfarin. At time of questionnaire, 214/256 (84%) of patients who replied had an estimated CHA2DS2-VASc score ≥2. Interestingly, only 45% of the Preop AF patients received warfarin, compared with 35% of the Postop AF patients and 7% of the No AF patients (). No patient with a CHA2DS2-VASc score ≥5 received warfarin.
Discussion
The main finding of this study is that AF was an independent risk factor for late cardiovascular morbidity and mortality in the patients undergoing CABG surgery, regardless of whether it was present before surgery or occurred postoperatively. The findings are in accordance with previous, separate studies of preoperative [Citation1,Citation6] and postoperative AF.[Citation2,Citation8,Citation16] To our knowledge, however, the present study is the first in which all three groups of patients are compared in the same patient cohort. In the present study, we have been able to link the increased mortality to specific death causes: the risk of cerebral ischaemic death was dramatically increased in both AF groups of patients, as was death due to heart failure and sudden death. Moreover, the resemblance between the two AF groups is striking in this respect. What is the possible explanation for this?
We have previously shown that an episode of postoperative AF is a risk indicator for future AF – in one study, at least one-quarter of postoperative AF patients developed AF during a 6-year follow-up.[Citation11] In the present study, irregular pulse was a common symptom in the postoperative AF group at follow-up. The risks of cerebral ischaemia, sudden death and heart failure are increased in non-surgical patients with AF [Citation4,Citation5] and there is no reason to believe that these risks are lower in CABG surgery patients. Altogether, these findings suggest a relationship between the increased mortality in both AF groups and the development and continued perseverance of AF. Notably, some important confounders such as left ventricular diastolic function and medication are not sufficiently controlled for, and causality cannot be inferred from the present study design. Anticoagulation is at present the only therapy that has been proven to reduce mortality in AF patients.[Citation17] Despite clinical evidence and guidelines, only 25–50% of non-surgical AF patients with thromboembolic risk factors are offered antithrombotic therapy and many patients who receive therapy discontinue their treatment.[Citation18,Citation19] In our study, 82% of patients in the Preop AF group had an estimated CHA2DS2-VASc score of ≥2 at follow-up, but only 45% received warfarin, and the proportion of Postop AF patients receiving anticoagulation was even lower. Given the high incidence of death and hospitalization because of ischaemic stroke in this group of patients, it seems reasonable to argue that the number of Preop and Postop AF patients receiving anticoagulation should be higher.
Regarding the group of patients with postoperative AF, the possibility to detect and diagnose AF in daily clinical practice is reduced because of the often silent nature of the arrhythmia. About one-third of AF patients are asymptomatic and in many cases AF is only diagnosed after a complication such as a stroke or congestive heart failure.[Citation20] As we now know that postoperative AF patients carry an increased risk of future AF,[Citation11] these findings support a better follow-up strategy for patients with postoperative AF, as well as better compliance to existing guidelines for patients with known AF. Clinical trial evidence is required to guide optimal decisions regarding long-term anticoagulation in the postoperative AF group of patients. In the immediate postoperative period, strategies of rhythm or rate control should be guided by symptoms since neither treatment has shown a net clinical advantage over the other.[Citation15]
Today, patients with preoperative AF undergoing coronary surgery can be offered concomitant AF ablation, according to guidelines.[Citation21,Citation22] Successful perioperative ablation in cardiac surgery patients increases the freedom of AF and may improve myocardial function [Citation23] and quality of life,[Citation24] but an increased risk of pacemaker implantation has also been observed.[Citation25] The findings of this study confirm the detrimental impact of AF on long-term mortality and cardiovascular morbidity, and support the need for larger studies of long-term effects of concomitant AF surgery.
Limitations
Preoperative AF patients were identified based on patient history and documented AF in previous ECG recordings. It is therefore possible that some patients with preoperative paroxysmal AF were undetected and wrongly classified as No AF patients. Likewise, intermittent recordings of heart rhythm postoperatively could miss some episodes of postoperative AF. The prevalence and incidence of preoperative and postoperative AF in the study cohort was, however, in accordance with previous studies.
From an epidemiological perspective, the study’s cohort size was limited, especially the size of the preoperative AF group. The results of the regression analysis must therefore be interpreted with caution, and the findings of this study should be regarded as hypothesis-generating. While the findings suggest an underuse of anticoagulation in AF patients undergoing coronary surgery, the management of the individual patients may have been based on clinical data unknown to the investigators.
Data from the Swedish National Cause of Death Register were complete, but different methods were used to establish the cause of death, and therefore cause of death may be incorrectly recorded in this database. The observed difference between the groups concerning cardiovascular and non-cardiovascular death cannot, however, be easily explained by poor validity of the cause of death data. The follow-up regarding survival and cause of death was complete, but 22% of surviving patients did not answer the questionnaire. The results should therefore be interpreted with caution, as the data are subjective reports and have not been validated by comparison with patient records or prescriptions.
Conclusion
Patients with pre- or postoperative AF undergoing CABG surgery have an increased long-term mortality and increased risk of cerebral and cardiovascular death compared with patients in sinus rhythm. Anticoagulation treatment based on stroke risk evaluation is important in this group of patients, and long-term effects of concomitant AF surgery in preoperative AF patients need further evaluation.
Notes on contributors
Espen Fengsrud is a consultant in cardiology and electrophysiology at Úrebro University Hospital.
Anders Englund is associate professor at Karolinska Insitute and the head of Arrhythmia Center, Stockholm.
Anders Ahlsson is a cardiac surgeon and the head of the Division of Cardiorespiratory diseases and Diagnostics at Úrebro University Hospital.
Acknowledgements
We would like to thank personnel from Clinical Research Support at Örebro University Hospital for their valuable help in collecting data.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
Research Committee, Örebro University Hospital, 136/04.
References
- Ngaage DL, Schaff HV, Mullany CJ, et al. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2007;133:182–189.
- Ahlsson A, Bodin L, Fengsrud E, et al. Patients with postoperative atrial fibrillation have a doubled cardiovascular mortality. Scand Cardiovasc J. 2009;43:330–336.
- Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861.
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988.
- Quader MA, McCarthy PM, Gillinov AM, et al. Does preoperative atrial fibrillation reduce survival after coronary artery bypass grafting? Ann Thorac Surg. 2004;77:1514–1522.
- Saxena A, Dinh D, Dimitriou J, et al. Preoperative atrial fibrillation is an independent risk factor for mid-term mortality after concomitant aortic valve replacement and coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;16:488–494.
- Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–1618.
- Thoren E, Hellgren L, Granath F, et al. Postoperative atrial fibrillation predicts cause-specific late mortality after coronary surgery. Scand Cardiovasc J 2014;48:71–78.
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429.
- Ahlsson A, Fengsrud E, Bodin L, et al. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg 2010;37:1353–1359.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972.
- Lonnerholm S, Blomstrom P, Nilsson L, et al. Effects of the maze operation on health-related quality of life in patients with atrial fibrillation. Circulation. 2000;101:2607–2611.
- Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921.
- Phan K, Ha HS, Phan S, et al. New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2015;48:817–824.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867.
- Rowan SB, Bailey DN, Bublitz CE, et al. Trends in anticoagulation for atrial fibrillation in the U.S.: an analysis of the national ambulatory medical care survey database. J Am Coll Cardiol. 2007;49:1561–1565.
- Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–2605.
- Savelieva I, Camm AJ. Clinical trends in atrial fibrillation at the turn of the millenium. J Intern Med. 2001;250:369–372.
- Ahlsson A, Jideus L, Albage A, et al. A Swedish consensus on the surgical treatment of concomitant atrial fibrillation. Scand Cardiovasc J. 2012;46:212–218.
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Europace. 2012;14:528–606.
- Stulak JM, Dearani JA, Daly RC, et al. Left ventricular dysfunction in atrial fibrillation: restoration of sinus rhythm by the Cox-maze procedure significantly improves systolic function and functional status. Ann Thorac Surg. 2006;82:494–500.
- Forlani S, De Paulis R, Guerrieri Wolf L, et al. Conversion to sinus rhythm by ablation improves quality of life in patients submitted to mitral valve surgery. Ann Thorac Surg. 2006;81:863–867.
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399–1409.