Abstract
Objectives. Transcatheter aortic valve implantation (TAVI) is an established treatment for high-grade aortic valve stenosis in patients found unfit for open heart surgery. The method may cause cardiac conduction disorders requiring permanent pacemaker (PPM) implantation, and the long-term effect of PPM implantation remains ambiguous. Design One hundred sixty-eight patients who underwent TAVI from 2008 to 2012 were included. Patient characteristics, ECGs and PPM data were collected through medical records. Kaplan–Meier plots and Cox regression analysis were performed. Results. Forty subjects were excluded, leaving 128 patients for final inclusion. 41 (32%) received a PPM (mean age 82 vs. 80 in patients without PPM, p = .06) within 30 days of the TAVI procedure. Median follow-up was ∼4 years and 37 (29%) died. One-year mortality was 14% for non-PPM patients vs. 2% in PPM patients, and mortality at 5yrs 70% vs. 54%, respectively. Kaplan–Meier survival analysis showed higher mortality in patients without PPM (p = .008). In multivariate survival analysis significant variables were: No PPM (HR 2.6; CI 1.1–6.2; p = .03), chronic obstructive pulmonary disease (HR 2.4; CI 1.2–5.0; p = .02) and either pre- or post-procedural chronic or paroxystic atrial fibrillation (HR 2.3; CI 1.2–4.7; p= .02). Conclusion. TAVI-patients with a PPM had better survival than patients in whom a PPM was not implanted.
Introduction
Transcathether aortic valve implantation (TAVI) has become a common treatment for patients with severe symptomatic aortic valve stenosis (AVS). Due to increasing patient age and the arteriosclerotic nature of the disease, AVS is often accompanied by comorbidities or systemic dysfunctions causing many patients to be inoperable by conventional surgical aortic valve replacement (SAVR). By avoiding sternotomy and cardiopulmonary bypass, TAVI has been shown to effectively reduce both symptoms and mortality, when compared to conventional surgery. However, TAVI also results in a higher frequency of new-onset cardiac conduction disorders (CCD).[Citation1–3] Recently, Nazif et al. showed a reduction in TAVI-induced CCDs over time,[Citation4] but data addressing permanent pacemaker (PPM) treatment in the setting of TAVI patients remains limited. Since a PPM treatment is a costly procedure and not without risk, unnecessary implantations should preferably be avoided. Furthermore, due to the relative infancy of the treatment, long-term mortality of TAVI-subjects still has to be evaluated. The primary aim of this study was to evaluate the effect of PPM on mortality rates.
Material and methods
In this single-center, retrospective study, consecutive patients with symptomatic AVS, who were treated at Odense University Hospital, Odense, Denmark, with TAVI between 25 March 2008 and 24 September 2012 were enrolled. The patients had previously been selected for TAVI in accordance with guidelines [Citation5] by a highly specialized multidisciplinary team consisting of a cardiologist, anesthesiologist and a thoracic surgeon. The treatment decision was based on clinical history, including comorbidity, transthoracic/transesophageal echocardiography and in most cases the results of a multidetector computed tomography. A 12-lead ECG was taken when the patient was admitted for the TAVI procedure, and post-procedural ECGs were taken on a daily basis. Both the Medtronic CoreValve Revalving System (MCRS; Medtronic Inc., Minneapolis, MN) and the Edwards-Sapien XT Valve (Edwards Lifesciences, Irvine, CA) were used, with sizes ranging from 23 to 31 mm. Femoral arteries were the primarily used access points, while subclavian arteries were chosen in a few cases. Both general and local anesthesia was used. A temporary pacemaker electrode was placed in all patients for at least four days, during which telemetry was also used.
Absolute indications for PPM implantation were: 3rd degree atrioventricular (AV)-block, 2nd degree AV-block type 2, and sick sinus syndrome. Prophylactic indications were new left bundle branch block (LBBB) in combination with 1st degree AV-block.
Data on baseline characteristics, pre-procedural echocardiography, TAVI-procedure details, and the clinical indications for PPM were collected together with pre-procedural and daily post-procedural ECGs for at least four days, from electronic medical records. Baseline characteristics included the following: age, gender, body mass index, smoking status (never, prior, current), family history of heart disease, hypertension, hypercholesterolemia, diabetes mellitus, prior acute myocardial infarction, prior percutaneous coronary intervention or coronary artery bypass graft, chronic obstructive pulmonary disease, stroke and/or transient cerebral ischemic attack, renal disease (defined as a estimated glomerular filtration rate below 60 ml/min), heart failure (defined as left ventricular ejection fraction (EF) < 50%), logistic Euroscore I, New York Heart Association (NYHA) class and implanted valve brand. The ECGs were blindly analyzed by two authors with regard to atrial fibrillation or flutter, PR-, QRS- and QTc-intervals, right and left bundle branch blocks (RBBB, LBBB), left anterior and posterior hemiblocks (LAHB, LPHB) and the degree of AV block. In case of discrepancies between the two authors, the ECG was adjudicated by a committee of local experts. In case of pace rhythm, no further ECG-analysis was performed. Patients, who received a PPM within 30 days of TAVI were identified through the electronic medical records. Pacemaker data were collected from the hospital’s pacemaker-database or from follow-up notes in the electronic medical records, if the patient was referred from another hospital. The pacemaker data from the patient’s last follow-up was used. Data on survival status were collected from the Danish Civil Personal Registration System.[Citation6] The clinical outcomes were: (1) PPM implantation within 30 days, (2) PPM use, defined as ventricular pacing of more than 3% of the time and (3) all-cause death after the TAVI-procedure. The 3% cutoff was chosen partly due to the results and the assumption that a healthy individual would have a similar pacing percentage due to asymptomatic variation in the heart rhythm. To minimize the risks of a type II error mistake, a p-cutoff value of <.2 for the univariate “first-pass” analysis, was used based on the fairly low number of subjects in the study as well as the comparably high number of variables.
Descriptive data for categorical variables are expressed as counts and percentages. Continuous variables are expressed as mean ± standard deviation or median with range. Categorical values were compared using the chi-square or Fisher’s exact test and continuous variables were compared using Student’s t-test or Wilcoxon–Mann–Whitney test, as appropriate.
Unadjusted survival data were analyzed with the Kaplan–Meier curves and compared between groups (with and without PPM) with the log-rank test adjusted for age. To analyze the prognostic effect of variables on mortality at follow up, univariate and multivariate Cox regression analyses were carried out. Variables tested were age, sex, BMI, PPM within 30 days of TAVI procedure, baseline and the last post-operative ECG characteristics, baseline comorbidities (hypertension, diabetes mellitus, hypercholesterolemia, prior coronary artery disease, chronic obstructive pulmonary disease, renal insufficiency, prior stroke, prior transient ischemic attack, chronic heart failure), logistic Euroscore, EF, smoking status (non/former/current), family history of cardiovascular disease and prosthesis brand. Variables with p < .2 in the univariate Cox regression analyses were included in the multivariate model. Variable selection for the multivariate Cox regression analysis was done with the backwards elimination method, each time excluding the one variable with the highest p-value. The variables age, gender and ejection fraction were included despite p > .2 due to their known negative effect on prognosis. All data were analyzed using Stata/IC 13.1 (College Station, TX).
Results
One hundred sixty-eight patients had a TAVI procedure done during the inclusion period. Seventeen patients were excluded due to a PPM prior to the TAVI treatment (). Another 23 patients were excluded for the following reasons: no baseline ECG available (n = 20), no post-TAVI ECGs available (n = 2), or death within 24 hours of TAVI (n = 1). The baseline characteristics of the 128 patients included in the study are shown in . The mean age was 81 years, and 100 patients received a Medtronic CoreValve whereas 28 had an Edwards-Sapien XT Valve. The ECGs are shown in . A total of 10 patients had pace rhythm on the last ECG. The PQ- and QRS-intervals were 182ms ±37 and 107ms ±27 (mean ± SD) at baseline and increased post-operatively and peaked on the fourth day at 203 ms ±56 and 133ms ±42 respectively (p = .0001 and p < .0001). After the TAVI procedure, 19 patients had developed 1st degree AV-block, and 48 patients developed new LBBB (43%). Excluding patients who had 1st degree AV-block and LBBB prior to the TAVI procedure showed no significant differences in the baseline ECGs when comparing Medtronic CoreValve recipients to Edward-Sapien XT recipients. However, there was a significant difference in the post-procedural PQ-interval (197ms vs. 175ms, p = .02), and a trend towards longer QRS-interval (134 ms vs. 115 ms, p = .08).
Figure 1. Study population and groups. PPM: permanent pacemaker. VPP: ventricular pacing percentage.
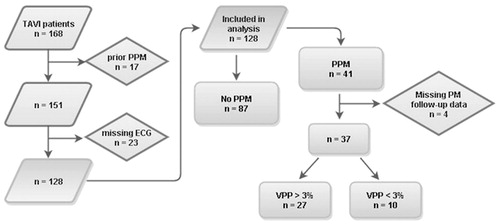
Table 1. Baseline and procedural data of population.
Table 2. ECG Prior to and in the 4 days after TAVI.
Medtronic CoreValve having the higher values
A total of 41 (32%) subjects received a PPM within 30 days after the TAVI procedure (average of 9 days, range 2–20). Among patients who had a Medtronic CoreValve, 38 (38%) underwent a PPM implantation compared to 3 (11%) of the patients who got an Edwards-Sapien XT Valve (p = .006). An absolute indication for PPM was present in 19 patients: 3rd degree AV-block (n = 15), 2nd degree AV-block type 2 (n = 3) and sick sinus syndrome (n = 1). Prophylactic PPM was indicated in 22 patients due to new 1st degree AV-block combined with LBBB. Of patients with PPMs, a higher percentage were male (58% vs. 39%; p = .04) and older (82 vs. 80, p = .06). No significant difference in the frequency of baseline comorbidities between those with and without PPM was found. However, the groups differed in both baseline and post-procedural ECGs (). The PQ- (p < .001) and QRS-intervals (p = .04) were longer, and the prevalence of RBBB was higher (p = .002) in PPM recipients when looking at the baseline ECGs. Even after exclusion of patients who were paced on the ECGs after TAVI, this was also true for the PQ-interval (p < .001), the prevalence of 1st degree AV-block (p = .001) and RBBB (p = .01) in the post-procedural ECGs.
Table 3. Pre- and post-TAVI ECG differences between pacemaker recipients.
Forty-one patients received a PPM, but data on the use of PPM (ventricular pacing percentage) were missing in 4. Of the 37 patients, 10 (27%) had a ventricular pacing percentage <3%, while the remaining 27 (73%) paced >12% of the time. There was no difference in the use of PPM in terms of indications for PPM implantation: 4 of 17 (24%) in the group with absolute indication for PPM paced <3% compared to 6 of 20 (30%) in the group with prophylactic indication (p = .9).
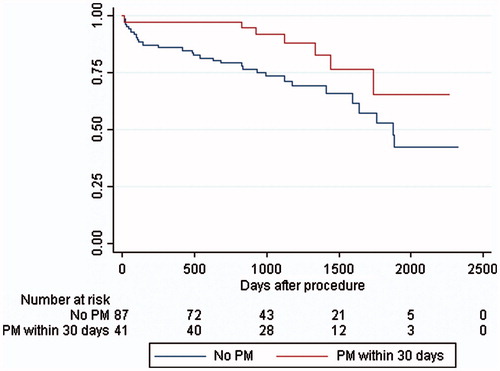
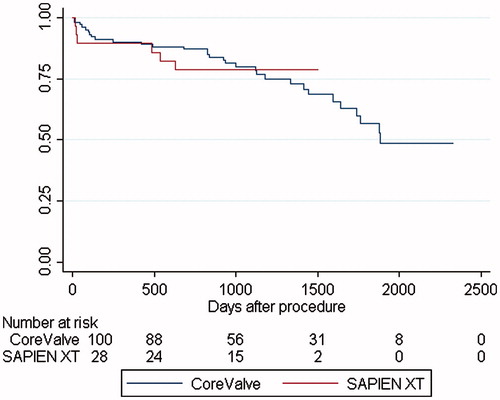
Median follow-up time or time to death for all 128 subjects was 1387 days (range: 691–2335 days). During the entire period, 37 (29%) patients died of whom 7 (19%) had received a PPM. The mortality rate for patients without PPM was 4.6% at 30 days and 14.5% at one year, with a yearly steady increase to 21.7%, 37.3%, 56.8% and 71.1%, whereas patients with a PPM rated 2.4%, 2.4%, 2.5%, 10.0%, 27.8% and 53.8%, with a significant difference at one (p = .05), two (p = .007) and three years (p = .01). Kaplan–Meier survival analysis () revealed an age-adjusted increased long-term all-cause mortality in subjects who did not receive PPM (p = .008). In the multivariate survival analysis using Cox’s regression model (), significant variables were: no PPM (HR 2.6; CI 1.1–6.2; p = .03), chronic obstructive pulmonary disease (HR 2.4; CI 1.2–5.0; p = .02) and either pre- or post-procedural chronic or paroxystic atrial fibrillation (HR 2.3; CI 1.2–4.7; p = .02). LBBB was not a significant marker for mortality. Four patients died within 30 days of the procedure (12, 13, 17, and 21 days, respectively) and of these one had received a PPM. Excluding these four patients did not change the results above: no PPM (HR 2.7; 1.0–6.8; p = .04), chronic obstructive pulmonary disease (HR 2.3; CI 1.0–5.1; p = .04) and atrial fibrillation (HR 2.7; CI 1.3–5.7; p = .009). When comparing the CoreValve to the Sapiens, our data showed no significant difference in mortality (p = .996), but it should be noted that the Sapiens XT was introduced later into the clinic resulting in a much shorter follow-up time ().
Table 4. Multivariate cox regression analysis with all-cause death as outcome.
Discussion
Our most surprising and somewhat controversial finding is that a post-TAVI PPM implantation is associated with a significant lower all-cause long-term mortality rate. In prior studies, PPM has either been found to have no effect or a negative effect on long-term survival rates.
In general, we found an impaired atrioventricular and intraventricular conduction after TAVI. Baseline PQ-intervals (182 ms) and QRS-intervals (107 ms) were almost identical to Boerlage-van Dijk et al.’s [Citation7] study of 121 patients (182 and 106 ms, respectively) while the pre-discharge ECG was comparable to our last PQ-interval at 200 ms and QRS-interval at 132 ms (compared to 199 and 128 ms, respectively). Frequencies of BBBs were also very similar between our and Boerlage-van Dijk et al.’s patients (pre-TAVI: RBBB 12% vs. 12%; LBBB 13% vs. 12%, post-TAVI: RBBB 8% vs. 6%; LBBB 55% vs. 58%), whereas only 22% received PPM implantation.
New onset LBBB in our study was 43%, which is consistent with Franzoni et al.’s [Citation8] study of 238 patients, where 27% of Edward Sapiens recipients and 50% of Medtronic CoreValve recipients had new onset LBBB, considering 78% of our patients received the Medtronic CoreValve. Franzoni et al, also showed a lower frequency of 1st degree AV-block and LBBB in recipients of a Edward Sapiens valve compared to Medtronic CoreValve (14% vs. 7%, p = .001 and 50% vs. 14%, p = .001, respectively). The apparent postoperative decrease in RBBB in our study (from 15 to 10 patients) is due to censoring of paced rhythm. Khawaja et al. [Citation9] looked at 243 patients, who received CoreValve prosthesis with baseline ECG characteristics similar to the patients in our study (baseline PQ-interval: 190ms and QRS-interval: 105 ms) and comparable post-operative conduction changes (1st degree AV-block 28%, LBBB 55%, RBBB 8%). In conclusion, the ECG changes in our patients seem to be comparable to what has been reported in other studies.
Our rate of PPM implantation was higher than the meta-analysis by Siontis et al.,[Citation10] who reported a median of 28% for the Medtronic CoreValve and 6% for Edwards-Sapien (compared to our 38% and 11%, respectively), perhaps due to cautious implantations on relative indications when the procedure was new. The reported baseline predictors of PPM implantation were male sex, 1st degree AV-block, left anterior hemiblock, and RBBB as well as intraprocedural AV-block. This is partly congruent with our findings in that we observed a higher prevalence of post-procedural 1st degree AV-block and RBBB in PPM recipients. Siontis also reported male gender to be associated with an increased risk for PPM implantation, whereas we did not find the difference statistically significant in the multivariate regression analysis (p = .5). Specific conduction disturbances were only reported in a few of the reviewed articles (from 10% to 25% depending on type), and therefore make it hard to interpret and comment in detail.
We found that PPM recipients were older than those who did not have a PPM. This is in line with the observations by D’Ancona et al.,[Citation11] who found age to be an independent predictor of PPM implantation in a study of 358 patients (HR 1.8; p = .05), who received Edwards Sapien prostheses with a lower PPM implantation rate of 6%. In a large German study of 1147 TAVI patients by Ledwoch et al.,[Citation12] a non-significant association between PPM implantation and age >80 years was shown (p = .08). This finding could be explained by a clinical assumption of increasing conduction abnormalities with higher age.
In agreement with our results, the Medtronic CoreValve prosthesis has been associated with increased conduction abnormalities and subsequent PPM implantation, possibly due to its self-expanding method of implantation as compared to the balloon-expandable Edward-Sapiens valve,[Citation1,Citation10,Citation13–15] but in a subgroup analysis the mortality does not differ.
In evaluation of the use of PPM, a confounding finding was that the pacemaker did pace <3% of the time in 27% of the relevant patients. Previously, it has been shown that TAVI-induced LBBB does not persist in as many as 40% of the patients 30 days after the procedure.[Citation4] The 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy as well as other studies have concluded that the need for permanent pacing in TAVI-patients drop with time at rates of up to 50%.[Citation15–17]
Our study showed lower rates of all-cause mortality throughout the first 4 years compared with data from Barbanti et al.’s study [Citation18] of 353 CoreValve recipients (56% female) with a lower PPM implantation rate of 24%. In that study the annual mortality rates were 21%, 29%, 38%, 48% and 55% even though patients with a PPM prior to TAVI (11%) were included. We found that implantation of a PPM within 30 days of TAVI showed a reduction in all-cause mortality, when compared with TAVI-patients who did not receive a PPM. This is surprising as the patients who had a PPM actually were older and primarily males. PPM recipients also had a significantly higher post-operative EF, but after adjustment a PPM was still associated with improved long term survival. This is a rare finding, but Urena et al. [Citation19] made a similar discovery in their multicenter analysis of 1556 subjects with fewer CoreValve recipients (45%) and PPM implantations (15%) with a mean follow-up of 22 ± 17 months. They found an insignificantly lower all-cause mortality rate in PPM recipients compared to non-PPM subjects (logrank p = .15), but a significant difference when looking at sudden cardiac death or death from an unknown cause (HR, 9.3, p = .02, logrank p = .03) which persisted after censoring short-term deaths. Their indication for PPM was stricter and elusive to compare since it was at the discretion of the physician in the presence of LBBB with 1st degree AV-block not expected to normalize. The study was also unable to see any negative effects of heart failure status, but found paroxystic/chronic fibrillation to be an independent predictor of unexpected death, all of which is consistent with our results. Opposed to this, the PARTNER trial [Citation20] concluded that patients with a new PPM had the same cardiovascular mortality rate after 1 year (7.6% vs. 8.4%, p = .7), but higher rates of all-cause mortality or repeated hospitalization (42% vs. 32%, p = .003), compared to patients who did not receive a PPM. De Carlo et al. [Citation21] concluded that being conservative with PPM implantation (24% before discharge) showed no difference in 1-year mortality rates between groups based on PPM status. However, in that study six patients received a PPM after 30 days and were included in the PPM-group. The PARTNER trial also showed that PPM patients have poorer evolution of left ventricular EF even though there are published case reports showing this to be treatable.[Citation22] A recent analysis of 833 patients who received a CoreValve prosthesis showed no difference in all-cause mortality (1 year: 16% vs. 15%, p = .8; 2 year: 16.9% vs. 16.3%, p = .8) in PPM recipients.[Citation23] A most humble and speculative explanation for these results is that while most patients recover from their post-TAVI conduction abnormalities, a few might have an increased risk of developing serious acute conduction abnormalities later on. Thus, they might develop 3rd degree AV-block, and if this happens to a patient without a PPM, it could become fatal. The problem is yet to identify these patients since we found no difference in the PPM dependency between the groups based on absolute and relative indication for PPM.
This study suffers from the limitation of being a retrospective cohort study. The study population is relatively small, but compared to other studies with a long follow-up time. There is an intrinsic problem when finding predictive factors that are used as clinical indicators for PPM implantation. It should also be noted that the PM-settings are assumed to be set to pace as little as possible, but would be more accurate with an active reassessment of patients’ CCD, where the pace rate would be turned down while an ECG is performed.
Patients receiving a post-operative PPM after TAVI do not have an increased risk of mortality and in fact demonstrated a lower all-cause long-term mortality in this study. A possible explanation is that we have treated a number of patients with prophylactic PPM, and therefore caught a few whose cardiac conduction progressed into higher grade abnormalities. Our study do provide some reassurance for physicians treating post-operative TAVI patients with ambiguous cardiac conduction changes, in that giving a PPM does not seem to increase mortality risk. In order to find out whether a liberal approach to PPM has a positive effect on mortality, one might consider a randomized clinical trial where one group receives PPM on absolute and relative indications and the other on absolute indications only.
Acknowledgments
We thank Fredrik Olson for assistance with data gathering, and Maria T. Lauritsen for help with analyzing ECGs.
Disclosure statement
No support in the form of grants, equipment or drugs have been supplied by third parties. The study complies with the Declaration of Helsinki. All authors have no conflicts of interest to declare.
References
- Erdogan HB, Kayalar N, Ardal H, et al. Risk factors for requirement of permanent pacemaker implantation after aortic valve replacement. J Card Surg. 2006;21:211–215. discussion 6–7.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607.
- Roten L, Stortecky S, Scarcia F, et al. Atrioventricular conduction after transcatheter aortic valve implantation and surgical aortic valve replacement. J Cardiovasc Electrophysiol. 2012;23:1115–1122.
- Nazif TM, Williams MR, Hahn RT, et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. 2014;35:1599–1607.
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–2496.
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25.
- Boerlage-Van Dijk K, Kooiman KM, Yong ZY, et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37:1520–1529.
- Franzoni I, Latib A, Maisano F, et al. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. Am J Cardiol. 2013;112:554–559.
- Khawaja MZ, Rajani R, Cook A, et al. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve Collaborative). Circulation. 2011;123:951–960.
- Siontis GC, Juni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64:129–140.
- D'Ancona G, Pasic M, Unbehaun A, et al. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2011;13:373–376.
- Ledwoch J, Franke J, Gerckens U, Kuck KH, Linke A, Nickenig G, et al. Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: analysis from the German transcatheter aortic valve interventions registry. Catheter Cardiovasc Intervent. 2013;82:E569–E577.
- van der Boon RM, Houthuizen P, Urena M, Poels TT, van Mieghem NM, Brueren GR, et al. Trends in the occurrence of new conduction abnormalities after transcatheter aortic valve implantation. Catheter Cardiovasc Intervent. 2015;85:E144–152.
- Koplan BA, Stevenson WG, Epstein LM, et al. Development and validation of a simple risk score to predict the need for permanent pacing after cardiac valve surgery. J Am Coll Cardiol. 2003;41:795–801.
- European Society of C, European Heart Rhythm A, Brignole M, Auricc A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013;15:1070–1118.
- Bjerre TJ, Loh PH, Cholteesupachai J, et al. Reevaluation of the indications for permanent pacemaker implantation after transcatheter aortic valve implantation. J Invas Cardiology. 2014;26:94–99.
- Pereira E, Ferreira N, Caeiro D, et al. Transcatheter aortic valve implantation and requirements of pacing over time. Pacing Clin Electrophysiol. 2013;36:559–569.
- Barbanti M, Petronio AS, Ettori F, et al. 5-Year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Intervent. 2015;8:1084–1091.
- Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233–1243.
- Dizon JM, Nazif TM, Hess PL, et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart. 2015;101:1665–1671.
- De Carlo M, Giannini C, Bedogni F, et al. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J. 2012;163:492–499.
- Osmancik P, Stros P, Herman D, et al. Cardiac resynchronization therapy implantation following transcatheter aortic valve implantation. Europace. 2011;13:290–291.
- Mouillet G, Lellouche N, Yamamoto M, et al. Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve devices: results from the FRANCE 2 Registry. Catheter Cardiovasc Interv. 2015;86:E158–E166.