Abstract
Transcatheter mitral valve (MV) intervention has emerged as an effective treatment option for symptomatic severe mitral regurgitation (MR) in patients considered to be inoperable or at high operative risk for surgical MV surgery. In primary mitral regurgitation, surgical repair is the standard of care. Transcatheter edge-to-edge MV repair with the MitraClip system has the largest clinical experience to date and offers a sustained clinical benefit in selected surgical high-risk patients. Surgery for secondary MR remains a challenge. Indications and the preferred surgical procedure remain controversial, mainly because of high recurrence rate of MR and the absence of evidence for survival benefit after surgery. Secondary MR is currently the most common indication for MitraClip use in Europe. Many registries show the safety of this procedure and improvements in patient symptoms and quality of life after 1 year, but most patients still have considerable residual MR. Other transcatheter MV repair devices are still in their early experiences. However, durability, safety, and possible damage of adjacent cardiac structures remain important concerns. Future directions for treatment of patients with secondary MR will depend on outcomes from the clinical trials in progress, whatever the use of transcatheter techniques is expected to expand substantially in the next years. This review aims to provide an overview of transcatheter MV interventions, emerging from surgical concepts, including leaflet repair, chordal replacement, and annuloplasty, and to discuss the challenges they face and future directions in achieving successful clinical application.
Introduction
Mitral valve (MV) disease is the most common valvular heart disease particularly due to its association with coronary artery disease with a prevalence of more than 10% in people aged older than 75 years.[Citation1] Regardless of its etiology, severe mitral regurgitation (MR) is associated with progressive left ventricular (LV) dysfunction and congestive heart failure, ultimately leading to high rates of morbidity and mortality. Current guidelines recommend surgery for moderate-to-severe or severe MR in patients with symptoms or evidence of LV dysfunction. MV repair or replacement improves functional performance and survival, but determination of optimal timing of these therapies can be clinically challenging.
MR is divided into either primary (a structural abnormality of the MV apparatus) or secondary (a disease of the left ventricle, which interferes with the function and integrity of the MV apparatus) MR.[Citation2,Citation3] Treatment of various valve disorders is dependent on the underlying etiology, pathophysiology, and natural history of each disorder.
Euro Heart Survey data revealed that almost half of symptomatic patients hospitalized with severe MR are not referred for surgery, mainly because of advanced age, comorbidities, and LV dysfunction.[Citation4] When MR is secondary to underlying LV dysfunction, the benefit of surgery is controversial.[Citation5] Therefore, patients with secondary MR and high surgical risk are more frequently denied surgery and referred to palliative management, carrying a poor short- and long-term prognosis.
The recent development of less invasive transcatheter mitral repair provides an additional therapeutic option for some high-risk and inoperable patients.[Citation6] Detailed patient and valvular pathology assessments as well as detailed insights into benefits and drawbacks of these emerging technologies are essential for appropriate utilization of medical, surgical, and transcatheter therapies in our expanding armamentarium.[Citation7]
This review highlights the clinical presentation, current, and future management for patients with primary and secondary MR. Transcatheter MV replacement technology is only sparely addressed and is beyond the scope of this review.
Mitral valve apparatus physiology
The MV is a complex apparatus integrated in the left ventricle, including the annulus, the leaflets, the chordae, the papillary muscles, and the ventricle itself ().[Citation8,Citation9] The mitral valve has anterior and posterior leaflets, which are separated by the anterior and the posterior commissure. The leaflets are inserted on the circumference of the mitral annulus in continuity with the aortic annulus and the left and right fibrous trigones. The circumflex coronary artery, coronary sinus, aortic valve, and bundle of His are all close to the mitral valve. The posterior MV leaflet usually consists of three discrete scallops, designated P1, P2, and P3. The corresponding segments of the anterior leaflet are designated A1, A2, and A3. The mitral leaflets receive chordae tendineae from the anterolateral and posteromedial papillary muscles. Primary chordae are attached to the free edge of the valve leaflet, and secondary chordae are attached to the ventricular surface of the leaflet. Beyond its obvious hemodynamic function to ensure forward cardiac output, the MV apparatus plays a fundamental role in the functional integrity of the left ventricle. Discontinuation of the mitral-ventricular continuity results in maladaptive remodeling and impaired left ventricular performance.[Citation10] Furthermore, the anterior leaflet acts as a rudder during the cardiac cycle to redirect flow from the lateral wall in diastole towards the outflow tract in systole.[Citation11] These factors seem to explain superior left ventricular performance and survival advantage following MV repair compared with valve replacement.[Citation12]
Figure 1. Mitral valve anatomy. The mitral valve comprises of the anterior and posterior leaflets, which are separated by the anterior commissure (AC) and the posterior commissure (PC). The leaflets are inserted on the circumference of the mitral annulus in continuity with the aortic annulus and the left and right fibrous trigones. mitral valve. Reprint with permission from Verma et al. [Citation9].
![Figure 1. Mitral valve anatomy. The mitral valve comprises of the anterior and posterior leaflets, which are separated by the anterior commissure (AC) and the posterior commissure (PC). The leaflets are inserted on the circumference of the mitral annulus in continuity with the aortic annulus and the left and right fibrous trigones. mitral valve. Reprint with permission from Verma et al. [Citation9].](/cms/asset/e224c691-1e42-4bd5-950a-738c8a3a4958/icdv_a_1248482_f0001_c.jpg)
Primary mitral regurgitation
In primary MR, regurgitation occurs due to biological changes in the MV leaflets and chordae. The most common cause of primary MR is degenerative MV disease. The spectrum of disease ranges from fibroelastic deficiency – resulting in leaflet prolapse or a flail leaflet segment due to ruptured chordae tendinae, most commonly of the middle portion of the posterior leaflet – to Barlow’s disease with excessive tissue and prolapse of both anterior and posterior leaflets.[Citation8] Both conditions can lead to leaflet prolapse and chordal elongation or rupture, representing the spectrum of degenerative mitral valve disease.[Citation8]
Patients with severe primary MR have an excess mortality rate of 6% per year compared with the expected survival rate. Even in asymptomatic patients the disease is associated with a high morbidity, with 10-year incidence of cardiac events (cardiac death, atrial fibrillation of and heart failure) of 33%.[Citation13] During 10 years follow-up, 90% of patients with severe MR will either have died or undergone surgical repair because of developing heart failure symptoms.[Citation14] Sudden death might also occur with an incidence of 2% per year and is responsible for about a quarter of deaths in patients receiving medical treatment.[Citation15]
Current treatment
Medical therapy
Medical therapy does not alter the natural history of patients with severe primary MR. For patients who are symptomatic with severe primary MR, diuretics and afterload reduction might relieve symptoms of heart failure, but the only curative treatment is mechanical correction by surgery or intervention.[Citation2]
Surgery
Surgical repair and replacement are invasive therapies available to patients with MV disease, that aim to completely eliminate MR. Surgical repair is the preferred treatment for patients with primary MR and is associated with better outcomes than mitral replacement.[Citation16] MR can usually be repaired by either resection of the flail and prolapsing leaflet segment or by reconstructive techniques using artificial polytetrafluoroethylene (ePTFE) chords. To select the most appropriate repair technique, a complete understanding of the underlying degenerative etiology, anatomical lesions, and leaflet dysfunction (excess or restricted leaflet motion) is mandatory.
In 1983, Dr. Alain F. Carpentier presented new systematic approach for reconstructive valve surgery.[Citation17] This paper outlined the basic pathophysiological classification of MV lesions and provided a functional approach based on four general principles for how to successfully and reproducibly repair MR, particularly from degenerative myxomatous MV disease. First, repair must restore an adequate surface of coaptation of both leaflets in systole. Second, full leaflet motion should be restored or preserved. Third, to prevent progressive dilatation, an annuloplasty ring or band should be used to reinforce the repair by stabilizing the annulus. Last, the surgeon should ensure that no more than trace-to-mild MR is present at the completion of the repair. Intraoperative transoesophageal 2D and 3D transoesophageal echocardiography is applied to guide the procedure and confirm a good result.[Citation18] In patients with isolated prolapse of the posterior middle scallop (P2), which is encountered in the majority of patients with degenerative MR, repair usually involves limited resection of this scallop, including the removal of the limited number of adjacent chordae (, Panel A). The remaining segments of the posterior leaflet are then sutured together to restore leaflet continuity. If excessive posterior-leaflet tissue is present, the height of the posterior leaflet is reduced by incisions in basal portion of posterior leaflet, followed by reapproximation of the leaflet remnants to the annulus (“sliding plasty”). This is critical to avoid systolic anterior motion of the anterior leaflet after valve repair.[Citation8] If residual prolapse remains despite tailored resections of abnormal tissue, then transfer of secondary chords to the free margin of the same segment, or chordal transposition from one segment to another is performed.
Figure 2. Surgical repair techniques. Quadrangular or triangular incision of isolated prolapse of P2 (Panel A). The remaining parts of the posterior leaflet (P1 and P3) are approximated and an annuloplasty ring is used to stabilize the annulus, thus preventing progressive dilatation. Chordae tendineae replacement (Panel B) with an artificial Gore-Tex (ePTFE) sutures to substitute ruptured or elongated chordae. Edge-to-edge repair (Panel C) is performed by sewing the anterior and posterior leaflets together at the central points of their middle segments to correct anterior or bileaflet prolapse while leaving a double-orifice mitral valve. Reprint with permission from Verma et al. [Citation9].
![Figure 2. Surgical repair techniques. Quadrangular or triangular incision of isolated prolapse of P2 (Panel A). The remaining parts of the posterior leaflet (P1 and P3) are approximated and an annuloplasty ring is used to stabilize the annulus, thus preventing progressive dilatation. Chordae tendineae replacement (Panel B) with an artificial Gore-Tex (ePTFE) sutures to substitute ruptured or elongated chordae. Edge-to-edge repair (Panel C) is performed by sewing the anterior and posterior leaflets together at the central points of their middle segments to correct anterior or bileaflet prolapse while leaving a double-orifice mitral valve. Reprint with permission from Verma et al. [Citation9].](/cms/asset/b4c89173-73f5-47d7-9c70-3c605c4b634a/icdv_a_1248482_f0002_c.jpg)
An alternative and complementary paradigm of “respect rather than resect” tissue has become popular in recent years, and is based on the use of ePTFE neochordae to reconstruct support of the free edge of prolapsing segments, and “displacing” abnormal excess tissue into the ventricle to ensure a good surface of coaptation without systolic motion of the anterior leaflet (, Panel B).[Citation19] Depending on the individual anatomy, the neochordae are anchored on the anterior or posterior papillary muscle tips in their fibrous portion, respecting a policy of not crossing the midline or individual native chordae in order to prevent excess traction on the leaflet margin. In the case of significant excess posterior leaflet height, the neochordae are made short enough to displace the prolapsing segment into the left ventricle, to ensure a large surface of leaflet coaptation while preventing anterior leaflet displacement in the outflow tract. Early results have been encouraging and no or limited leaflet resection in combination with ePTFE loops is now a preferred technique in many centers today.
Alfieri proposed a technique to approximate the anterior and posterior leaflet edges at the site of the regurgitation, applicable for both primary and secondary MR (, Panel C).[Citation20] This technique ensured permanent coaptation at the regurgitation site and when combined with an annuloplasty ring was shown to be effective in eliminating regurgitation and its recurrence. The non-physiological, dual-orifice geometry of the mitral valve after an edge-to-edge repair remains a concern in regards to long-term durability, and is a topic of several investigations. Edge-to-edge repair also imposes a tethering force on the valve leaflets, which can potentially cause adverse long-term remodeling of the tissue and clip dehiscence.[Citation21] De Bonis et al. reported a high rate of recurrence rate following surgical edge-to-edge repair without annuloplasty and advocated routine use of adjunct annuloplasty for this technique.[Citation22]
Regardless of the leaflet and chordal techniques employed, a prosthetic ring or band annuloplasty that restores the normal circumference and shape of the mitral valve to match the available leaflet tissue is a mainstay of all repair procedures.[Citation8] The fibrous skeleton of the heart is not contiguous around the posterior aspect of the mitral annulus, so long-standing regurgitation associated with ventricular and atrial enlargement leads to pathologic dilatation of the mitral annulus, particularly along the posterior aspect of the valve. Regardless of the type of annuloplasty (complete/partial – rigid/semi-rigid, or flexible rings), most surgeons measure the surface of the anterior leaflet with a sizer to estimate the appropriate ring size for the amount of leaflet tissue. Failure to perform an annuloplasty at the time of MV repair is one of the strongest predictors of failure resulting in recurrent moderate or severe MR.[Citation23,Citation24] This may have important implications for percutaneous techniques that primarily attempt to address the prolapsing segment without concomitantly changing the shape of the annulus.
The repair is assessed initially by visual inspection and by injecting saline through the mitral valve to look for regurgitation (the “saline test”), and then by intraoperative transesophageal echocardiography after the patient is weaned from cardiopulmonary bypass. Patients should not leave the operating theater with more than trace-mild MR on transesophageal echocardiography.[Citation8,Citation25]
MV repair is associated with an operative mortality less than 3% and nearer 1% in high-volume centers.[Citation26] The most common cause of death is heart failure. The most important late complication of MV repair is recurrent MR, which within 20 years follow-up may occur in 10% of patients and may lead to reoperation in approximately 6% of patients.[Citation25]
Transcatheter mitral valve repair
Although surgery is the gold standard intervention in patients with severe primary MR, a rationale exists for the use of transcatheter MV therapies. Many patients who need treatment are elderly with several comorbidities, so surgery is high risk or even contraindicated, leading to its underuse in clinical practice.[Citation4] Transcatheter MV repairs are a new class of therapies that could achieve reduction in MR, without the risk of surgery.
Various technologies of intervention, at different stages of investigation, have been introduced to minimize the surgical trauma and related risks with reasonable early, short-term success, but outcomes that are not comparable to surgical repair. These approaches can mainly be categorized into leaflet repair, annuloplasty, and chordal replacement (). A number of transcatheter MV repair innovations are in pipeline undergoing preclinical validation. The following section will focus on the few technologies having longer-term safety and efficacy documentation. A more comprehensive review of the emerging technologies can be found elsewhere.[Citation27]
Table 1. Transcatheter mitral valve repair devices.
Leaflet repair
In current practice, transcatheter MV intervention is mainly limited to the edge-to-edge repair technique with MitraClip (Abbott Vascular Inc, Santa Clara, CA) therapy. This technique reproduces the surgical Alfieri technique of edge-to-edge leaflet repair by clipping together the free edges of valve leaflets (). The procedure is conducted on beating heart using fluoroscopic and transoesophageal echocardiographic guidance.[Citation28,Citation29]
Figure 3. MitraClip (Abbott Vascular Inc, Santa Clara, CA) is a percutaneous mitral repair based on Alfieri edge-to-edge repair (see , panel C), designed for both degenerative and secondary MR.
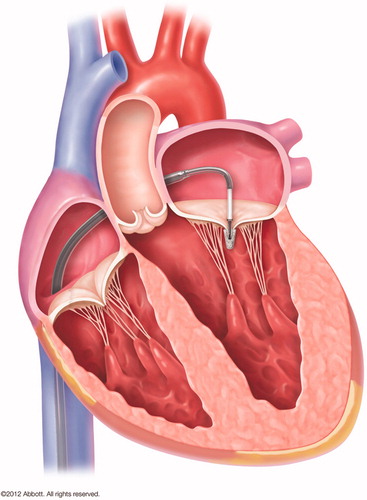
The MitraClip is only approved for use in patients with primary MR who have severe symptoms and are at high or prohibitive risk of surgery in United States, but is approved for clinical use for both primary and secondary MR in Europe.[Citation30] More than 30 000 patients worldwide have been treated with this procedure to date. The randomized EVEREST II trial compared MitraClip with surgery and showed a higher percentage of patients with significant residual MR in those who had received MitraClip compared with surgery (MR grade ≥2: 57% versus 24%, p < 0.001).[Citation28] At 5-years follow-up, the need for surgery was significantly higher in patients who had received MitraClip compared with surgery (28% versus 9%).[Citation31]
Current European [Citation2] and American [Citation3] guidelines state that MitraClip therapy can be considered in patients with symptomatic, severe, primary MR who fulfill the echocardiographic criteria of eligibility, are judged inoperable or at high surgical risk by a heart team. However, there is relevant concern that the suboptimal long-term results of surgical edge-to-edge repair without an annuloplasty may also apply to the percutaneous technique. One clinical study has proposed annular remodeling effect following mitral clip implantation.[Citation32] Further evidence should be accumulated in randomized studies comparing MitraClip with surgery.
Mitral annuloplasty
Transcatheter annuloplasty devices are at an early development phase. Even though the technology may carry significant potential as adjunct procedure for repair of primary MR in the future, the devices have so far primarily been evaluated for treatment of high-risk patients with secondary MR (see this section).
Chordal replacement
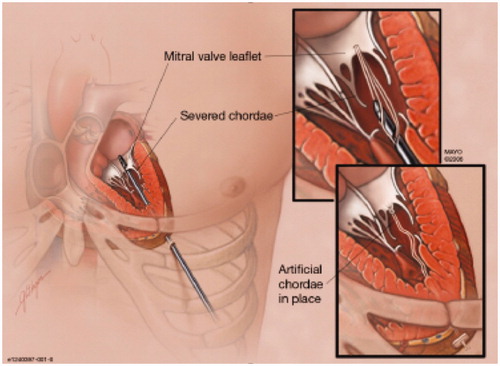
Replacement of ruptured chordae with ePTFE sutures via catheters is a potentially appealing technique to correct primary MR, since long-term efficacy is supported by surgical evidence. In the past 5 years, two transcatheter techniques of placing artificial chords by a transapical approach have been developed. Neochord (Neochord, Minnetonka, MN) was the first to report transapical beating heart implantation of ePTFE chordal loops on the MV leaflet to correct flail or prolapse ().[Citation33] Weber et al. [Citation34] investigated the ideal site of ventricular anchoring for artificial neochordae and speculated based on anatomical measurements from cardiac magnetic resonance imaging that attachment to the ventricular apex instead of the papillary muscle tips may increase the risk of systolic anterior motion and chordal dehiscence from the leaflet. Jensen et al. [Citation35] reported that the site of the chordal insertion had little influence on peak forces, although the rate of change of force in the chord were significantly higher following apical fixation compared to papillary muscle tip fixation. Early results from the TACT trial demonstrated acute procedural success in 26 of the 30 patients, with only 17 patients with <2 + grade MR at 30 d. In the initial phase of the study, two patients underwent reoperation for a failed repair.[Citation33] After adding more sutures and fixating the sutures more postero-laterally in the left ventricle, the acute procedural success rate improved. However, long-term freedom from recurrent MR might be also governed by the extent of annular dilatation and lack of annular support in these patients.
Future directions
Reconstructive valve surgery will probably remain the standard of care in low-risk or intermediate-risk patients with primary MR in the next 5 years. Transcatheter interventions will provide a satisfactory palliation in high-risk and inoperable patients. An obvious next step in the future will be to combine repair techniques by adding percutaneous annuloplasty to either leaflet repair by Mitraclip or chordal replacement aiming at reproducing long-term durable results of surgery. Transcatheter valve implantation is also being developed for more complex cases of primary MR though beyond the scope of this review.[Citation6]
Secondary mitral regurgitation
Secondary MR occurs when the MV leaflets are normal, but left ventricular dilation results in leaflet tethering and annular dilation that prevents leaflet coaptation. A vicious cycle of volume overload occurs, leading to progressive annular dilation, myocardial thinning, cavity dilation, increased left ventricular wall stress, and increased leaflet tethering, resulting in progressive MR and heart failure.[Citation11]
Secondary MR includes both ischemic and non-ischemic functional MR. In ischemic MR, there is tethering of the posterior leaflet which is associated with an akinetic or hypokinetic posteromedial segment of the left ventricular wall, resulting in a posteriorly directed jet of MR into the left atrium. In non-ischemic functional MR, displacement of both papillary muscles due to global LV systolic dysfunction results in a central jet of MR.
Patients with chronic ischemic MR have a worse prognosis than primary MR. Increasing severity of coronary artery disease and left ventricular dysfunction is associated with worse outcome.[Citation11]
Current treatment
Medical therapy
First-line treatment for patients with chronic secondary MR consists of guideline-directed medical therapy for left ventricular dysfunction, including angiotensin-converting enzyme inhibitors, beta-blockers, and aldosterone antagonists.[Citation2,Citation3] For patients with chronic secondary MR and left-bundle branch block and/or left ventricular dyssyncrony, cardiac resynchronization therapy might improve left ventricular function and reduce MR severity.
Surgery
Surgery for secondary MR remains a challenge. Operative mortality is higher than in primary MR and the long-term prognosis is worse – partly due to the more severe comorbidities. Furthermore, indications and the preferred surgical procedure remain controversial, mainly because of the persistence and high recurrence rate of MR after valve repair and lack of evidence that surgery prolongs life.[Citation36–38] Therefore, current guidelines advice intervention should only be undertaken if severe symptoms are unresponsive to optimum medical therapy.[Citation2] Correction of secondary MR by repair techniques with an undersized annuloplasty is the most common surgical procedure performed for functional MR, either alone or as a part of a complex repair.[Citation36,Citation38–40] The rationale of this procedure using very small (size, 24–26 mm) rings is that undersizing the mitral annulus will result in increased leaflet coaptation and decreased regurgitation. Although this procedure does not fully address the ventricular causes of secondary MR, it is relatively easy to perform and will lead to symptomatic improvement. There is general consensus that MV annuloplasty should be performed with complete rigid rings and not partial and flexible bands that might not sufficiently increase leaflet coaptation.[Citation40,Citation41] Several etiology-specific rings are commercially available; however, differences in ring design have not yet demonstrated significant differences in clinical outcome.[Citation42]
Although a restrictive ring annuloplasty can abolish MR acutely with short-term symptomatic improvement, the recurrence rate is high (11–33% of patients in a year) and improvement in long-term survival has not been shown.[Citation36,Citation38,Citation43,Citation44]
Several adjunct surgical strategies have been proposed for treatment of secondary ischemic MR. The first is based on the relocation of posterior papillary muscle tip to counteract posterior papillary muscle tethering which has been identified as a main mechanism of ischemic MR.[Citation45,Citation46] The second procedure consists of the cutting of second-order chordae tendineae to the anterior leaflet which has been shown to improve coaptation and reduce MR.[Citation47,Citation48]
Recently, a randomized trial assessing MV repair versus replacement in secondary MR showed equivalent clinical outcomes but a lower recurrence of MR with replacement after 2 years.[Citation36,Citation37] Additionally, these studies identified inferobasal aneurysm or dyskinesis, severe leaflet tethering, significant ventricular dilation, or depressed ejection fraction as predisposing factors for recurrence with repair.[Citation44] Hence, these patients should be considered for adjuvant subvalvular remodeling or valve replacement.
Transcatheter mitral valve repair
Transcatheter repair of secondary MR may have a higher clinical impact than primary MR, as conventional surgery is performed at higher risk in most patients and the outcomes of surgical repair are less predictable. On one hand, a successful transcatheter technology for secondary MR could enable early correction of MR, and potentially block the progressive momentum towards progressive LV remodeling and heart failure.[Citation49,Citation50] On the other hand, secondary MR may just be a marker of poor LV function, and the fundamental question remains about the efficacy of any valve intervention for patients with secondary MR.[Citation11]
Leaflet repair
Secondary MR is currently the most common indication for MitraClip use in Europe. Many registries show the safety of this procedure and improvements in patient symptoms and quality of life after 1 year, but most patients still have some residual or recurrent MR.[Citation29,Citation51,Citation52]
European guidelines [Citation2] state that the MitraClip procedure can be considered as an option – mostly to improve symptoms – in patients who are symptomatic despite optimum medical therapy, fulfill the echocardiography criteria of eligibility, are judged to be inoperable or at high surgical risk by a team of cardiologists and cardiac surgeons. Catheter-based MV repair for secondary MR did not receive recommendation in the 2014 ACC/AHA valvular heart disease guidelines.[Citation3]
Non-randomized comparisons with historical controls have suggested that MitraClip might have a benefit (compared with medical therapy) in reducing the need for readmission to hospital and improvement of patient survival in patients with severe secondary MR and left ventricular dysfunction.[Citation53,Citation54] However, data from randomized trials are needed to show that intervention that aim to interrupt the dysfunctional cycle of volume overload from MR results in improved long-term outcome.
In an attempt to expand the indications, ongoing clinical trials are investigating the effectiveness of MitraClip in secondary MR patients beyond optimal medical therapy. The COAPT trial (www.clinicaltrials.gov, identifier NCT01626079) is enrolling high surgical risk patients in United States. The RESHAPE 2 (www.clinicaltrials.gov, identifier NCT01772108) and Mitra-FR study (www.clinicaltrials.gov, identifier: NCT01920698) are similar trials being conducted in Europe.
Mitral annuloplasty rings
Transcatheter annuloplasty devices have primarily been tested for secondary MR. The first generation includes coronary sinus annuloplasty (e.g. Carillon device, Cardiac Dimensions, Kirkland, WA) [Citation55,Citation56] in which an artificial, deformable device is implanted into the coronary sinus allowing post-implant cinching of the device leading to reduction in the circumference and septal-lateral dimensions of the mitral annulus (, Panel A). Tomographic imaging studies demonstrated that the location of the coronary sinus is basally displaced from the mitral annulus in most patients, resulting in the reduction in the left atrium and not the mitral annulus.[Citation57] Complications with compression of the circumflex artery, transient myocardial perfusion, and injury to the septal conduction system have challenged clinical implementation of this technology.[Citation55,Citation56]
Figure 5. Percutaneous mitral annuloplasty systems. Carillon (Cardiac Dimensions, Kirkland, WA) Indirect Annuloplasty (Panel A) is an implantable mitral annular constraint device percutaneously placed into the coronary sinus primarily designed for secondary MR (and potentially for primary MR). Cardioband (Valtech, Yehuda, Israel) Direct Annuloplasty (Panel B) is an annuloplasty band with plicating anchors on the atrial side of mitral annulus designed for secondary MR (and potentially for primary MR).
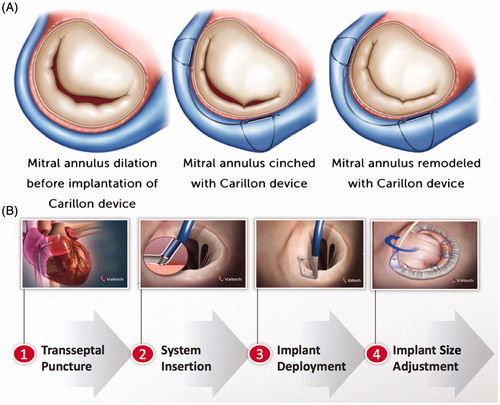
Cardioband (Valtech, Yehuda, Israel) is the only direct annuloplasty technique that best resembles surgical annuloplasty, in which a flexible annuloplasty band is delivered onto the posterior mitral annulus and attached with spiral anchors (, Panel B).[Citation58] Although appealing, it is unclear from pre-clinical or clinical trials if sufficient reduction in annular area can be achieved with a flexible band. Skepticism about the technology exists in the surgical community, since flexible partial rings have failed in these same patient groups when implanted surgically.[Citation41] However, in primary MR, it may prove to be a valuable adjunct catheter-based procedure to either leaflet repair by Mitraclip or chordal replacement (primary MR).
The Mitralign direct annuloplasty system is a more recent technology that replicates the Kay–Wooler commissuroplasty.[Citation59] In this procedure, a catheter is advanced retrogradely to the left ventricle and guidewires penetrate the mitral annulus into the left atrium, whereby pairs of pledgets are implanted in the commissures of the mitral annulus. Each pledget pair can be pulled together resulting in a segmental posterior annuloplasty. Six-month follow-up in 41 patients reported 12.2% mortality and 80% of the patients had ≥2 + MR severity,[Citation60] demonstrating suboptimal results similar to surgical commissural cinching.
Many companies have completed first-in-human transcatheter MV replacement; however, no devices are currently approved beyond compassionate use, and several others remain in preclinical development. These replacement devices vary in both access site and design, including technology for mitral annular and subvalvular anchoring. Detailed reviews of current and developing transcatheter MV replacement technologies have been reported recently.[Citation6]
Future directions
A combination of catheter-based interventions – e.g. MitraClip plus annuloplasty – could be considered for patients with secondary MR in the future. In parallel, further technological innovations will refine transcatheter MV replacement devices. As with surgery, the two techniques will probably be complementary: Repair being preferred at an early stage of the secondary MR with less leaflet deformation and complex anatomy and replacement in those with more severe MR due to greater leaflet tethering and ventricular dilation.
Discussion
As the population in the Western world continues to age, the prevalence of significant MR is expected to further increase. In high-volume heart-centers, the operative mortality for mitral intervention is <1%, and successful surgical repair improves long-term survival with 95% freedom from repeat surgery and >80% freedom from moderate to severe or severe MR at 20 years after surgery.[Citation25] Guideline recommendations to treat even asymptomatic patients with severe MR in heart valve centers of excellence that have high repair success rates and low surgical mortality might be realized over the next 5 years.[Citation30]
Nevertheless, in the real world, almost 50% of patients who meet current indications for MV surgery are not offered this therapy because of elevated surgical risk.[Citation4] In these high surgical risk patients transcatheter approaches for severe MR have emerged during the past decade as a viable, less invasive therapeutic alternative. The MitraClip system has proven reasonable safety and efficacy in high-risk patients and is already considered as an established part of the MV program in experienced centers. Clinical trials of MitraClip versus surgery in intermediate-risk patients are expected to begin within the next years. Future directions for treatment of patients with secondary MR will depend on outcomes from the clinical trials in progress, especially the COAPT trial, which will end patient enrollment this year. If these trials show that MitraClip is better than medical therapy regarding survival and freedom from readmission to hospital after treatment, then the indications for transcatheter techniques are expected to expand substantially. However, if findings from these trials are neutral or even negative, then the use of these techniques for secondary MR will naturally be slowed. Since the Mitraclip implantation in many patients is offered as a last therapeutic option beyond optimal medical therapy and CRT, it is plausible (and subgroup analyses from these trials may support) that patient referrals for transcatheter techniques (repair and replacement) will probably increase despite neutral study outcomes.
Other transcatheter MV repair devices are still in their early experiences. However, durability, safety, and possible disruption of adjacent cardiac structures remain important concerns.
It is important to emphasize that novel transcatheter techniques for the treatment of MR are not meant to replace surgical techniques in lower risk patients who are good candidates for surgery. Because various transcatheter MV interventions will be available in the future, it is of particular importance to acknowledge a very long-term surgical experience in order to understand how to tailor the right device strategy to the right patients. A multidisciplinary heart-team approach will play a crucial role for careful patient selection and clinical implementation of these emerging transcatheter interventions as a part of a successful and multi-modal MV program.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014. AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365.
- Mihaljevic T, Lam B-K, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201.
- Maisano F, Alfieri O, Banai S, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur Heart J. 2015;36:1651–1659.
- Vesely MR, Benitez RM, Robinson SW, et al. Surgical and transcatheter mitral valve repair for severe chronic mitral regurgitation: a review of clinical indications and patient assessment. J Am Heart Assoc. 2015;4:e002424.
- Carpentier A, Adams DH, Filsoufi F. Carpentier's reconstructive valve surgery. Riverport Lane: Saunders; 2010.
- Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med. 2009;361:2261–2269.
- Yun KL, Fann JI, Rayhill SC, et al. Importance of the mitral subvalvular apparatus for left ventricular segmental systolic mechanics. Circulation. 1990;82:IV89–I104.
- Levine RA, Hagege AA, Judge DP, et al. Mitral valve disease-morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710.
- Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007;31:267–275.
- Enriquez-Sarano M, Avierinos J-F, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883.
- Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417–1423.
- Grigioni F, Enriquez-Sarano M, Ling LH, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34:2078–2085.
- Enriquez-Sarano M, Schaff HV, Orszulak TA, et al. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995;91:1022–1028.
- Carpentier A. Cardiac valve surgery – the "French correction”. J Thorac Cardiovasc Surg. 1983;86:323–337.
- O'Gara P, Sugeng L, Lang R, et al. The role of imaging in chronic degenerative mitral regurgitation. JACC Cardiovasc Imaging. 2008;1:221–237.
- Perier P, Hohenberger W, Lakew F, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the “Respect Rather Than Resect” approach. Ann Thorac Surg. 2008;86:718–725.
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122:674–681.
- Nielsen SL, Timek TA, Lai DT, et al. Edge-to-edge mitral repair. Circulation. Am Heart Assoc J. 2001;104:I–29–I–35.
- De Bonis M, Lapenna E, Maisano F, et al. Long-term results (≤18 Years) of the edge-to-edge mitral valve repair without annuloplasty in degenerative mitral regurgitation: implications for the percutaneous approach. Circulation. 2014;130:S19–S24.
- Gillinov AM, Tantiwongkosri K, Blackstone EH, et al. Is prosthetic anuloplasty necessary for durable mitral valve repair? Ann Thorac Surg. 2009;88:76–82.
- Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003;107:1609–1613.
- David TE, Armstrong S, McCrindle BW, et al. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation. 2013;127:1485–1492.
- Gammie JS, O'Brien SM, Griffith BP, et al. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation. 2007;115:881–887.
- Espiritu D, Onohara D, Kalra K, et al. Transcatheter mitral valve repair therapies: evolution, status and challenges. Ann Biomed Eng 2016.
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406.
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052–1061.
- Nishimura RA, Vahanian A, Eleid MF, et al. Mitral valve disease-current management and future challenges. Lancet. 2016;387:1324–1334.
- Feldman T, Kar S, Elmariah S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854.
- Schueler R, Momcilovic D, Weber M, et al. Acute changes of mitral valve geometry during interventional edge-to-edge repair with the MitraClip system are associated with midterm outcomes in patients with functional valve disease: preliminary results from a prospective single-center study. Circ Cardiovasc Interv. 2014;7:390–399.
- Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol. 2014;63:914.
- Weber A, Hurni S, Vandenberghe S, et al. Ideal site for ventricular anchoring of artificial chordae in mitral regurgitation. J Thorac Cardiovasc Surg. 2012;143:S78–S81.
- Jensen H, Jensen MO, Waziri F, et al. Transapical neochord implantation: is tension of artificial chordae tendineae dependent on the insertion site? J Thorac Cardiovasc Surg. 2014;148:138–143.
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32.
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–353.
- Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188.
- Bolling SF, Deeb GM, Brunsting LA, et al. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg. 1995;109:676–682.
- Braun J, van de Veire NR, Klautz RJM, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85:430–436.
- Spoor MT, Geltz A, Bolling SF. Flexible versus nonflexible mitral valve rings for congestive heart failure: differential durability of repair. Circulation. 2006;114:I67–I71.
- Khamooshian A, Buijsrogge MP, de Heer F, et al. Mitral valve annuloplasty rings: review of literature and comparison of functional outcome and ventricular dimensions. Innovations. 2014;9:399–415.
- Magne J, Girerd N, Sénéchal M, et al. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short-term and long-term survival. Circulation. 2009;120:S104–S111.
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:752–761.e1.
- Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600–601.
- Nappi F, Lusini M, Spadaccio C, et al. Papillary muscle approximation versus restrictive annuloplasty alone for severe ischemic mitral regurgitation. J Am Coll Cardiol. 2016;67:2334–2346.
- Messas E, Guerrero JL, Handschumacher MD, et al. Chordal cutting: a new therapeutic approach for ischemic mitral regurgitation. Circulation. 2001;104:1958–1963.
- Borger MA, Murphy PM, Alam A, et al. Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2007;133:1483–1492.
- Auricchio A, Schillinger W, Meyer S, et al. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol. 2011;58:2183–2189.
- Grayburn PA, Foster E, Sangli C, et al. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation. 2013;128:1667–1674.
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011–2012 Pilot European Sentinel Registry. J Am Coll Cardiol. 2014;64:875–884.
- Suerder D, Corti R, Klersy C, et al. TCT-718 percutaneous mitral valve repair in functional mitral regurgitation: preliminary results from the of the Swiss nationwide investigator-initiated prospective MitraClip® registry (MitraSwiss). J Am Coll Cardiol. 2015;66:B293.
- Van den Branden BJL, Swaans MJ, Post MC, et al. Percutaneous edge-to-edge mitral valve repair in high-surgical-risk patients: do we hit the target? JACC Cardiovasc Interv. 2012;5:105–111.
- Velazquez EJ, Samad Z, Al-Khalidi HR, et al. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: a propensity-matched comparison. Am Heart J. 2015;170:1050–1053.
- Schofer J, Siminiak T, Haude M, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation. 2009;120:326–333.
- Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN trial. Eur J Heart Fail. 2012;14:931–938.
- Maselli D, Guarracino F, Chiaramonti F, et al. Percutaneous mitral annuloplasty: an anatomic study of human coronary sinus and its relation with mitral valve annulus and coronary arteries. Circulation. 2006;114:377–380.
- Maisano F, Taramasso M, Nickenig G, et al. Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J. 2015;37:ehv603–ehv825.
- Kay JH, Magidson O, Meihaus JE. The surgical treatment of mitral insufficiency and combined mitral stenosis and insufficiency using the heart-lung machine. Am J Cardiol. 1962;9:300–306.
- Nickenig G, Schueler R, Dager A, et al. Treatment of chronic functional mitral valve regurgitation with a percutaneous annuloplasty system. J Am Coll Cardiol. 2016;67:2927–2936.