Abstract
Objectives. Comparisons between remote magnetic (RMN) and manual catheter navigation for atrial fibrillation (AF) ablation have earlier been reported with controversial results. However, these reports were based on earlier generations of the RMN system. Design. To evaluate the outcomes of the most current RMN system for AF ablation in a larger patient population with longer follow-up time, 112 patients with AF (78 paroxysmal, 34 persistent) who underwent AF ablation utilizing RMN (RMN group) were compared to 102 AF ablation patients (72 paroxysmal, 30 persistent) utilizing manual technique (Manual group). Results. The RMN group was associated with significantly shorter fluoroscopy time (10.4 ± 6.4 vs. 16.3 ± 10.9 min, p < .001) but used more RF energy (64.1 ± 19.4KJ vs. 54.3 ± 24.1 KJ, p < .05), while total procedure time showed no significant difference (201 ± 35 vs. 196 ± 44 min, NS). After 39 ± 9/44 ± 10 months of follow-up, AF-free rates at 1year, 2 years and 3.5 years post ablation were 63%, 46% and 42% in the RMN group vs. 60%, 32% and 30% (survival analysis p < .05) in the Manual group, whereas clinically effective rates were 82%, 73% and 70% for the former vs. 70%, 56% and 49% for the latter (survival analysis p < .005). Conclusion. Differing from previous reports, our data from a larger patient population and longer follow-up time demonstrates that compared to manual technique, the most current RMN technique is associated with better procedural and clinical outcomes for AF ablation.
Introduction
Various mapping and catheter navigation techniques have been utilized to facilitate atrial fibrillation (AF) ablation and improve clinical outcome [Citation1]. Remote magnetic catheter navigation (RMN) technique is one of the most advanced methods that has been used for AF ablation for more than 10 years [Citation2,Citation3]. Several groups have published procedural and clinical outcomes of the RMN technique vs. those of the manual catheter technique, but with controversial results: while some of them reporting RMN gave outcome similar to manual method [Citation3–7], some recent reports suggested that RMN technique was associated with higher recurrence rates [Citation8,Citation9]. It is worth mentioning that almost all the published studies were based on clinical data using earlier generations of the RMN system, non-irrigated or first generation irrigated-tip ablation catheters, and non-steerable transseptal sheaths, including those published recently [Citation8,Citation6,Citation7]. Older generations of the RMN system had limitations in quickly and accurately responding to the operator’s command [Citation10]. The initial solid-tip RMN catheter had problems in char formation and inability to achieve efficacious lesions [Citation10], while the first generation irrigated-tip RMN ablation catheter had its limitations in uniformity of irrigation flow, effective thermal conductivity, etc [Citation3]. In addition, the available data had smaller patient groups and/or shorter follow-up times.
The aim of the present study was to evaluate the efficacy and clinical outcome of the RMN technique for AF ablation based on our data utilizing the latest generation of the RMN system on a larger patient population with longer follow-up time to provide our evidence to this important clinical issue.
Methods
Patients
Patient data were retrospectively collected. We started to use the most current RMN system (NiobeTM ES/Epoch Solutions, Stereotaxis, Inc. St Louis, MO, USA) for AF ablation from August 27, 2012 and through December 12, 2014, 112 consecutive patients underwent their first AF ablation under the NiobeTM ES system, 78 with paroxysmal AF and 34 with persistent AF. Data from these 112 patients (RMN group) were compared with those from 102 consecutive patients who underwent their first AF ablation in our center during the period from December 1, 2011 through October 29, 2014 using conventional manual technique (Manual group). Seventy-two of the Manual group patients were with paroxysmal AF and 30 with persistent AF.
During follow-up, 36 (32%) patients in the RMN group and 32 (31%) in the Manual group underwent a 2nd procedure for improvement of clinical outcomes and the procedural and follow-up data of these 2nd procedures were also included in the analysis. Some of the patients underwent a 3rd procedure and even 4th procedure, however, follow-up data after these procedures were not yet available and therefore they were not included.
All patients were symptomatic and at least one antiarrhythmic agent failed before they referred to our center for AF ablation. All patients continued their anticoagulation and antiarrhythmic drugs under the procedure. Informed consent to the treatment was obtained from each patient prior to the procedure. The study was performed in accordance with the principles outlined in the Declaration of Helsinki. Baseline characteristics of the two groups are shown in .
Table 1. Baseline characteristics of the RMN and manual groups.
The remote magnetic navigation system
The RMN system has been previously described in detail [Citation10]. In brief, the latest, i.e. the 4th generation of the Stereotaxis magnetic navigation system (Niobe ES) consists of two permanent magnets that placed on either side of the patient’s chest, generating a uniform magnetic field (0.08-0.10 T) to navigate the mapping and ablation catheter. Differing from earlier generations of the Niobe system, the Niobe ES system features rapid catheter navigation in response to the user’s commands in less than 250 ms. A specially designed, second generation of 3.5-mm irrigated-tip ablation catheter (Thermocool/C3/RMT, Biosense-Webster) was used. The RMT catheter is manufactured with a super-flexible distal shaft and 3 magnets embedded within the distal portion of the catheter, including one in the distal electrode. The catheter tip is remote-controlled via the RMN system to enable the operator to direct the catheter tip quickly and precisely to desirable locations in the cardiac chambers for mapping and allows stable contact to the tissue during ablation [Citation10]. An electro-anatomical mapping system (CARTO 3, Biosense-Webster, Inc) was integrated into the RMN system on the same screen (Odyssey VisionTM, Stereotaxis, Inc.), so that the operator could use his/her skill and experience for 3-dimentional mapping and linear ablation while using the RMN technique.
Electrophysiology procedure
A bipolar catheter for right ventricular pacing and a 10-polar catheter for coronary sinus pacing and electrogram recording were inserted via the right femoral vein. A third access was established in all patients via a transseptal puncture using a non-steerable sheath (SWARTZ SL0, St. Jude Medical, Inc.). In a subset of the patients (n = 29 or 26% for RMN group and n = 53 or 52% for the Manual group), a second transseptal puncture was made for introducing a circular catheter (Lasso, 20-polar 25-35 mm expendable, Biosense-Webster, Inc). In cases of double transseptal punctures, the second transseptal sheath used a steerable sheath (Agilis, St. Jude Medical Inc.).
In the RMN group, the reconstruction of a 3-dimentional map of the left atrium was most frequently performed using the RMT catheter (n = 83), or manually using the circular catheter (n = 29). A robotic device (V-driveTM and V-CASTM, Stereotaxis, Inc.) for advancing, retracting and rotating the transseptal sheath was utilized in all patients. In the Manual group, the 3.5-mm irrigated-tip ablation catheter (Navistar ThermocoolTM, Biosense-Webster) was used in 74 patients (73%), while 28 patients (27%) used a contact force catheter (SmartTouchTM, Biosense-Webster). The left atrial map was constructed using the manual ablation catheter (n = 49) or a circular catheter (n = 53). The steerable sheath was used in 53 of the Manual group patients to extend the reach of the distal shaft of the ablation catheter and to make smooth movement of the catheter along the ablation lines while keeping desirable contact with the tissue.
In both groups, efforts were made to reconstruct details of the pulmonary vein (PV) antrum, taking reference of 3-dimentional CT/MR image of the left atrium that was obtained within 1 week prior to the procedure in all the patients ( and ). The ablation strategy was to apply circumferential ablation lesions around the PV ostia as guided by the electroanatomical maps and the 3-dimentional CT/MR image of the left atrium ( and ). The amplitudes and morphologies of the electrograms were also used as reference, especially on the ridge between the left atrial appendage and the left-sided PVs and on the carina between the upper and the lower PVs. When the two PVs were clearly separated, linear ablations were also applied on the carina if electrograms were still recordable after the completion of the encircling line. Incomplete lines/ablation points were applied when the two PVs were very close to each other, when a 5th PV was present in between, or when electrical signals only recorded partially at the carina.
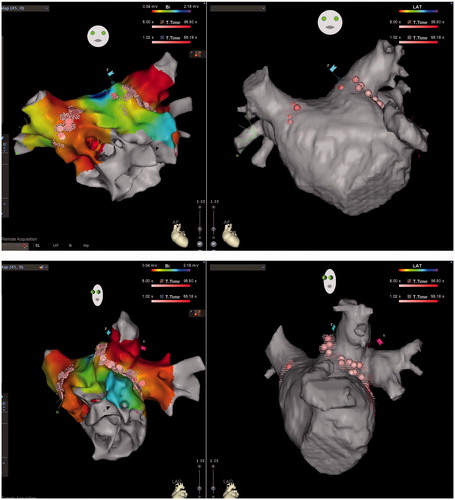
Figure 1. CARTO maps (left) and CT images (right) of the LA in a patient with paroxysmal AF who underwent 1st ablation in the RMN group, PA (upper) and LAO (lower) views, showing detailed reconstruction of the PV antrum as well as PV encircling ablation lines. Note linear ablation was even applied between the well-separated right-sided upper and lower PVs, but only encircling around the common left PV.
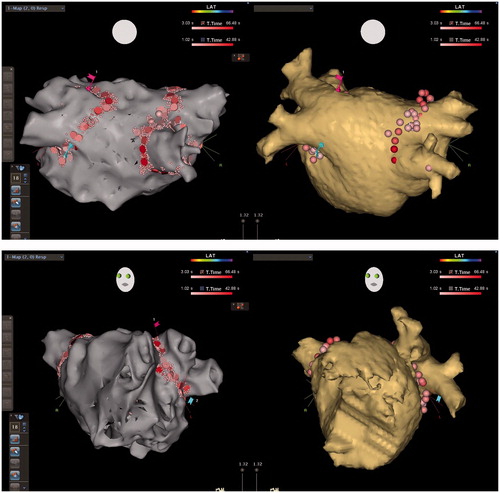
Figure 2. CARTO voltage maps (left) and CT images (right) of the LA in a patient with persistent AF who underwent 2nd ablation in the RMN group, AP (upper) and LAO (lower) views respectively, showing again detailed reconstruction of the PV antrum as well as reconnections to the antero-inferior right upper and antero-inferior right lower PV where RF applications were added to re-isolate these PVs. RF ablations were also conducted against recordable local electrograms along the ridge between the atrial appendage and the left PVs in order to abolish potential substrate for re-entry, even reconnection was not identified to the left PVs.
Standard RF generator settings included maximal temperature of 48 °C at a power of 30–35 W for the posterior wall, and 35–40 W for other areas. The irrigation flow rate was 17 mL/min during ablation. All patients received continuous Remifentanil (UltivaTM) intravenous injection for sedative and analgesic purposes at average 0.05 mcg/kg/min and average total dose 764 mcg in these patients, with individual adjustments according to symptom and response.
Ablation always started from left-sided PVs and then the right-sided ones. Upon completion of ablation around the right-sided PVs, started to recheck the left-sided PVs, and then recheck the right-sided ones before finishing. Procedure end-point was bidirectional electrical isolation of the PVs as verified by the following mapping and pacing techniques: 1) No recordable electrogram within or on the encircling ablation lines, as recorded using the circular catheter or the ablation catheter (entrance block). The feasibility of verifying entrance block using the ablation catheter was tested previously in our lab by condensed mapping using the RMN catheter and using the manual catheter: when no recordable electrogram was present the ablation catheter was replaced by a circular catheter which also showed no recordable signal in all patients. This led to the avoidance of a 2nd transseptal puncture and a circular catheter in most of our RMN group patients (n = 83 or 74%) and nearly half of our Manual group patients (N = 49 or 48%). 2) Pacing within the encircling area using either the ablation catheter or the circular catheter (at 10 mA, 2 ms pulse width) without capturing the atrium (exit block). 3) When residual electrograms were recorded within the PVs, especially in the left or right upper PVs, differential pacing outside the encircling area was also performed. If the electrograms were advanced to the pacing artifact, it suggested they were far-field signals near or from the pacing site. In cases with only one transseptal puncture, the CS catheter was used for differential pacing within the CS, in the right atrial appendage and even in the left atrium after inserting it into the left atrium along the ablation catheter through the septum. In patients with persistent AF, an additional roofline was also ablated if the two encircling lines were very close to each other. The completion of the roofline was verified by pacing and recording across the line. Other linear ablations were applied when macro-reentrant atrial tachycardias were detected/provocated during the procedure. Ablation of arrhythmogenic focus/foci was also conducted, when identified. The termination and non-inducibility of the tachycardia was the endpoint.
Follow-up
Patients continued antiarrhythmic and anticoagulation treatment during the blanking period of 3 months post procedure. Follow-up was performed at intervals of 3 months, 6 months, 1 year, and thereafter once a year. Evaluations at each follow-up were: 1) an out-patient visit to a cardiologist, 2) 3-7 days’ Holter recording, 3) cardiac CT/MR at first follow-up, and 4) a patient questionnaire including a) any symptomatic recurrence of AF and/or tachycardia; b) comparing with prior ablation, patient’s subjective judgments for his/her general condition, c) severity, d) frequency, and e) length of symptomatic recurrence of AF or tachycardia. The patient’s answer to each question was quantified into 11 grades from 0 to 10 for quantitative analysis, where grade 5 is no change. In addition to the scheduled follow-up visits/questionnaires, patients were instructed to contact a physician at anytime when suspecting AF recurrence or tachycardia for ECG and/or Holter documentation. Any AF or AT episode ≥30 s was defined as recurrence.
According to Holter and/or ordinary ECG recordings, physician’s judgments, and patient’s symptoms, results of follow-up were classified as 1) AF/AT-free, off or on antiarrhythmic drugs, 2) clinically improved: AF burden reduced ≥80% and/or improvement ≥ grade 8 according to the patient’s symptoms, 3) patients had little improvement, no effect, or developed atrial tachycardia. AF/AT-free and clinically improved together were referred as clinically effective in this study.
Statistical analysis
Study parameters are expressed as mean ± standard deviation (SD) and percentage (%). Differences in frequencies were assessed by using Chi-square test and differences in means were tested using Sdudent’s t-test or Mann-Whtney U test. Kaplan-Meier survival analysis was used to compare clinical outcomes during follow-up and Mantel-Cox Log rank test was used to examine the significance of the differences in survival rates between the two groups. AF/AT-free and clinically effective rates at 0.5 year, 1 year, 1.5 years, 2 years, 2.5 years and by the end of the follow-up at about 3.5 years post ablation were calculated to quantitatively present clinical outcomes. A p-value <.05 was considered statistically significant.
Results
Baseline characteristics of the patients are summarized in . There are no statistically significant differences between the groups in gender, age, AF history, left atrial size, prior cardioversion, persistent or paroxysmal AF, concomitant diseases or previous treatments of PCI, CABG and cardiac valvular surgeries. All patients reached the procedure endpoints. Procedural parameters are summarized in . Total procedure time was skin-to-skin time, including the time for cardioversion (n = 77), trans-esophageal echocardiography (n = 18) and rotation left atrial venography (n = 12) in the two groups. Around 80% of the femoral vein punctures, catheter insertion, and transseptal puncture were performed by physicians in-training, but the AF ablations per se were all performed by 3 experienced electrophysiologists: operators A, B and C performing 53 (36%), 24 (16%), and 71 (48%) RMN procedures and 54 (40%), 49 (37%) and 31 (23%) manual procedures, respectively.
Table 2. Procedural parameters of the RMN and manual groups.
The total fluoroscopy time was significantly shorter (p < .001) and total X-ray dose lower (p < .01) for the RMN group than for the Manual group. The total RF energy was greater (p < .05) and total ablation time was longer (p < .05%) for the RMN than for the manual group. There was no statistically significant difference in total procedure time between the two groups. Three patients (3%) in the Manual group had tamponade, all of which underwent acute pericardiocentesis without sequelae. One patient (0.9%) in the RMN group had symptomatic, significant left superior PV stenosis that required percutaneous trans-luminal angioplasty with a stent, and the intervention was successful.
When the available follow-up data was last collected, the patients were followed-up for 39 ± 9 (median 38) and 44 ± 10 (median 43) months in the RMN and the Manual groups, respectively. As shown in , the Kaplan-Meier survival analysis demonstrated that clinical outcomes were significantly better in the RMN group than in the Manual group, both in AF/AT-free survival (p < .05) and in clinically effective survival (p < .005). As shown in , both the AF/AT-free rates and the clinically effective rates at 0.5 year, 1 year, 1.5 years, 2 years, 2.5 years and by the end of the follow-up of about 3.5 years post ablation were significantly higher for the RMN group than for the Manual group, with the only exception that the difference between the two groups in AF/AT free at 1 year was not statistically significant. By the end of follow-up, 66% AF/AT free patients and 62% clinically effective patients were off anti-arrhythmic drugs in the RMN group, as compared to 65% AT/AF free patients and 56% clinically effective patients were off anti-arrhythmic drugs in the Manual group (NS).
Figure 3. Kaplan-Meier survival analysis for AF/AT-Free (upper) and Clinically Effective (lower), showing the RMN technique is associated with statistically better outcomes both for AF/AT-Free (p< .05) and for Clinically Effective (AF/AT-Free + Clinically Improved, p < .005) survivals. Note by the end of each survival curve are the remaining case number (%).
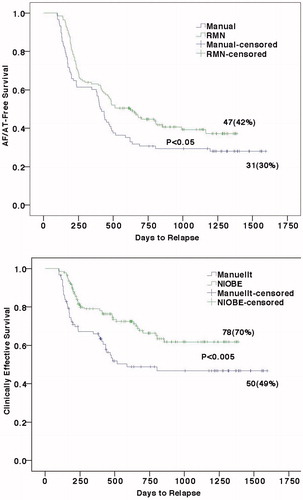
Table 3. Clinical outcomes of the RMN and manual groups.
Discussion
This study was aimed to evaluate the efficacy and clinical outcome of the latest RMN technique for AF ablation. To our knowledge, only two studies on procedural outcomes of AF ablation using the latest generation of the RMN system have newly been published [Citation11,Citation12]. Weiss et al. recently reported 1-year clinical outcomes of AF ablation using RMN technique vs. manual technique [Citation7]. However their patient population was a mixture of that using older RMN system (Niobe II) and the current RMN system (Niobe ES). Clinical outcomes of AF ablation purely utilizing the latest generation of the RMN system and its ablation catheter are so far lacking. In contrast to the previously published findings [Citation3–9], the current study with 214 consecutive AF patients and a mean follow-up time of 39/44 months demonstrated that AF ablation using the latest RMN technique was associated with statistically significant better clinical outcomes than using the conventional manual technique.
Procedural parameters
As shown in , our fluoroscopy time was significantly shorter and total X-ray dose lower than those in our Manual group, and than those in most of the previous published studies [Citation13,Citation3,Citation14,Citation8]. Notably Da Costa et al. [Citation12] and Jin et al. [Citation11] have recently reported even lower X-ray exposures using the latest system. In addition to total fluoroscopy time, we used also total X-ray dose to quantify the procedural X-ray exposure. We calculated also the total RF energy to quantify the RF output, which revealed that to isolate the PVs about 10000J more RF energy was needed using the RMN technique than using the manual technique, possibly due to the lower catheter-tissue contact force generated by the RMN system [Citation8].
Electrical PV isolation is the generally accepted procedural end-points [Citation1]. However, earlier clinical experience in segmental PV ablation had shown that electrical PV isolation is sometimes reached before the completion of PV encircling. PV isolation without completion of PV encircling often leads to AF recurrence and that was why segmental PV ablation was abandoned [Citation15]. In addition to PV isolation, we chose no recordable local electrogram within and on the encircling lines as one of our important procedural end-points to emphasize the importance of the completion of the encircling lines.
Clinical outcomes
Several clinical studies of AF ablation using the RMN technique have been reported and a majority demonstrated that RMN technique was associated with clinical outcomes similar to that of the conventional manual technique [Citation3–5,Citation7], which raised the question “is ‘as good as manual’ good enough”? [Citation16] There are also reports showing that the RMN technique was associated with lower success rate than the manual method [Citation9,Citation8]. Arya et al. reported that freedom from AF was reached at 66.4% using the manual vs. 57.8% using the RMN at 6 months of follow-up [Citation9]. Recently, Koutalas et al. again reported that at mean 30.3/27.3 months of follow-up, rate of freedom from AF was 40% in the RMN group as compared to 59.1% in the manual group with 70 patients with paroxysmal and persistent AF in each group [Citation8]. Thus, available clinical data showed controversial results when comparing outcomes of AF ablation utilizing the RMN and the manual technique. As mentioned above, all these studies used earlier generation RMN system.
Our patient data, ablation strategy and end-points are quite similar to those in the above-mentioned study [Citation8], but in our study the RMN technique was associated with statistically significant better clinical outcomes both in analyses of AF/AT-free and clinically effective survivals, as well as in success rates at different time points during long-term follow-up of more than 3.5 years. It is worth mentioning that all of our 3 operators had less than 1 year of experience for the RMN technique at the beginning of patient inclusion to this study, but their experiences in AF ablation utilizing the manual technique were all more than 10 years. In addition, a circular catheter, that had been proven to facilitate the achievement of PV isolation and thereby a better clinical outcome [Citation17], was used in 26% of the patients in the RMN group as compared to 52% in the Manual group. Despite these factors, our study demonstrated the RMN group was associated with a better clinical outcome, supporting the potential beneficial effect of the latest generation of the RMN technique. However, definitive verification of the clinical value of the most current RMN technique needs a randomized, prospective multi-center trail.
Success rate of AF ablation had earlier been reported as high as 85-90% [Citation18–20]. With accumulated worldwide experiences of AF ablation and long-term follow-up, the clinical outcomes are recognized to be not as optimistic as reported earlier, ranging from 40 to 68% reportedly using the RMN technique [Citation9,Citation8]. Long-term follow-up of 5 years showed that success rate was 20% after a single procedure and 45% after multiple procedures in long-standing persistent AF [Citation21], while the rate was 46.6% after a single procedure and 79.5% after multiple procedures in paroxysmal AF [Citation22]. Interestingly, a recent study revealed that reported high success rate was proven to be selectively cited, suggesting citation bias also contributed to the over optimistic clinical outcomes [Citation23]. Our follow-up data of the RMN group showed that freedom from AF/AT in 63%, 46% and 42% at 1, 2, and 3.5 years post ablation, which is inline with the currently reported clinical outcomes [Citation9,Citation8],including a recent survey on AF ablation by the European Heart Rhythm Association where success rate was 40.7% at 1 year follow-up [Citation24]. Remarkably, some of the reported success rates were from patients with a mixed follow-up time ranging from months up to several years [Citation8,Citation13,Citation14,Citation22], though in other studies they were more fairly calculated as success rates at 6 months [Citation9], 1 year [Citation7,Citation5,Citation24,Citation3], 2 years, 3 years [Citation5], etc., as we also did in our study. It was previously revealed that the success rate declines during long-term follow-up both in patients with paroxysmal [Citation22] and persistent AF [Citation21].Our patients also showed a similar trend of regression in success rate during follow-up, with rapid declining during the first one and a half years (, ).
Clinical outcome after AF ablation is a complicated entity that consists not only of patients who are free from AF or have no effect, but also of patients who are not yet totally free from AF but are significantly improved. In our study, we included an outcome category of clinically effective (free from AF/AT + clinically improved). Though the term “clinically effective” may be harder to define scientifically, it has important implications both for patients and clinicians and this category was also used in previously published clinical studies [Citation22].
Limitations
This is a non-randomized, retrospective study that is prone to bias and limitations. However, consecutive patients referred for AF ablation were included and they were assigned to receive either the RMN or the Manual AF ablation according to chronological order in the waiting list by planning nurses. The follow-up questionnaires were distributed, collected and registered by secretaries. The referring physicians carried out the vast majority of follow-up visits. None of the above persons had knowledge of the study. These factors should have partially minimized bias from the non-randomized design of this study.
In order to include more than 100 patients in each group, the patient inclusion periods were skewed between the two groups, with the start of inclusion for the manual group 9 months earlier than that for the RMN group, since we performed more RMN-procedures than manual ones by that time. However, considering the fact that all 3 operators had more that 10 years of experience in manual AF ablation, but they were all new for the RMN technique, this limitation should not have brought significant bias to our results.
It is known that clinical outcomes of AF ablation were better in paroxysmal AF than persistent AF [Citation21,Citation22]. We did not perform separate analysis of paroxysmal and persistent AF, for our purpose was to compare the efficacy and outcome of the RMN and Manual techniques. Since paroxysmal and persistent AFs were evenly distributed in the two groups, analysis of these patients together would not have essential influence on our results.
Conclusion
In contrast to previous reports, our study consisting of a larger patient population and longer follow-up period demonstrates that compared to conventional manual ablation technique, the most current RMN technique is associated with better procedural and clinical outcomes for AF ablation.
Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606.
- Pappone C, Vicedomini G, Manguso F, et al. Robotic magnetic navigation for atrial fibrillation ablation. J Am Coll Cardiol. 2006;47:1390–1400.
- Miyazaki S, Shah AJ, Xhaet O, et al. Remote magnetic navigation with irrigated tip catheter for ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:585–589.
- Proietti R, Pecoraro V, Di Biase L, et al. Remote magnetic with open-irrigated catheter vs. manual navigation for ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. 2013;15:1241–1248.
- Pezawas T, Ristl R, Bilinski M, et al. Single, remote-magnetic catheter approach for pulmonary vein isolation in patients with paroxysmal and non-paroxysmal atrial fibrillation. Int J Cardiol. 2014;174:18–24.
- Adragao PP, Cavaco D, Ferreira AM, Costa FM, Parreira L, Carmo P, et al. Safety and long-term outcomes of catheter ablation of atrial fibrillation using magnetic navigation versus manual conventional ablation: a propensity-score analysis. J Cardiovasc Electrophysiol. 2016;27(Suppl 1):S11–S16.
- Weiss JP, May HT, Bair TL, et al. A comparison of remote magnetic irrigated tip ablation versus manual catheter irrigated tip catheter ablation with and without force sensing feedback. J Cardiovasc Electrophysiol. 2016;27(Suppl 1):S5–S10.
- Koutalas E, Bertagnolli L, Sommer P, et al. Efficacy and safety of remote magnetic catheter navigation vs. manual steerable sheath-guided ablation for catheter ablation of atrial fibrillation: a case-control study. Europace. 2015;17:232–238.
- Arya A, Zaker-Shahrak R, Sommer P, et al. Catheter ablation of atrial fibrillation using remote magnetic catheter navigation: a case-control study. Europace. 2011;13:45–50.
- Di Biase L, Fahmy TS, Patel D, et al. Remote magnetic navigation: human experience in pulmonary vein ablation. J Am Coll Cardiol. 2007;50:868–874.
- Jin Q, Pehrson S, Jacobsen PK, et al. Efficacy and safety of atrial fibrillation ablation using remote magnetic navigation: experience from 1,006 procedures. J Cardiovasc Electrophysiol. 2016;27(Suppl 1):S23–S28.
- Da Costa A, Guichard JB, Maillard N, et al. Substantial superiority of Niobe ES over Niobe II system in remote-controlled magnetic pulmonary vein isolation. Int J Cardiol. 2017;230:319–323.
- Chun KR, Wissner E, Koektuerk B, et al. Remote-controlled magnetic pulmonary vein isolation using a new irrigated-tip catheter in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:458–464.
- Muntean B, Gutleben KJ, Heintze J, et al. Magnetically guided irrigated gold-tip catheter ablation of persistent atrial fibrillation–techniques, procedural parameters and outcome. J Interv Card Electrophysiol. 2012;35:163–171.
- Arentz T, Weber R, Burkle G, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–3063.
- Burkhardt JD, Di Biase L, Natale A. Remote magnetic navigation for atrial fibrillation ablation: is 'as good as manual' good enough. Europace. 2011;13:5–6.
- Vollmann D, Luthje L, Seegers J, et al. Remote magnetic navigation for circumferential pulmonary vein ablation: single-catheter technique or additional use of a circular mapping catheter?. J Interv Card Electrophysiol. 2014;41:65–73.
- Haissaguerre M, Jais P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–1417.
- Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544.
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081.
- Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the hamburg sequential ablation strategy. J Am Coll Cardiol. 2012;60:1921–1929.
- Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–2377.
- Perino AC, Hoang DD, Holmes TH, et al. Association between success rate and citation count of studies of radiofrequency catheter ablation for atrial fibrillation: possible evidence of citation bias. Circ Cardiovasc Qual Outcomes. 2014;7:687–692.
- Arbelo E, Brugada J, Hindricks G, et al. The atrial fibrillation ablation pilot study: a European survey on methodology and results of catheter ablation for atrial fibrillation conducted by the European heart rhythm association. Eur Heart J. 2014;35:1466–1478.
