Abstract
Objectives. This study evaluated angiographic success and in-hospital outcomes of percutaneous coronary intervention (PCI) with rotational atherectomy (RA) in patients with low left ventricular ejection fraction (LVEF). Design. Between January 2010 and March 2014, 272 consecutive patients with heavily calcified lesions underwent elective PCI with RA. Of these, 33 patients had LVEF ≤35% (low LVEF group), whereas 237 patients had LVEF >35% (preserved LVEF group). The primary endpoint was angiographic success and in-hospital major adverse cardiac events (MACE). MACE included death from any cause, postprocedure onset MI, emergency coronary artery bypass grafting, and target vessel revascularization. The secondary endpoints were MACE and the components within 30days after PCI. The components of MACE were evaluated. Results. Angiographic success, defined as <30% residual stenosis with thrombolysis in myocardial infarction flow 3 at final angiography, was achieved in all patients without fatal complications. Intra-aortic ballon pumping (IABP) was used significantly more frequently in the low LVEF group compared with the preserved LVEF group (15.2% vs. 2.1%, p = .003). There were no significant differences between groups regarding in-hospital and clinical outcomes within 30 days following PCI. Conclusion. If medications and mechanical support were appropriately performed, the angiographic success rate and in-hospital MACE rate of PCI with RA in patients with low LVEF could be expected to have good outcomes similar to those for patients with preserved LVEF.
Introduction
The indications of percutaneous coronary intervention (PCI) have grown dramatically since the introduction of the drug-eluting stent (DES) [Citation1–5]. However, even in the era of DES, the severe calcification of coronary artery lesions is associated with a high risk of target lesion revascularization (TLR) and restenosis [Citation6]. Previous studies have reported that severe calcification-disturbed stent expansion and inadequate stent expansion are major risk factors for restenosis after DES implantation [Citation7,Citation8]. Among the new devices, rotational atherectomy (RA) is uniquely effective in ablating heavily calcified lesions [Citation9–11]. The diamond-coated burr disperses the calcified plaque into micro-fragments. With an average diameter of 5 μm, these micro-fragments predominantly pass into the capillary circulation and are taken up by the reticuloendothelial system [Citation12,Citation13]. Potential complications of slowed flow and no flow are noted due to the down streaming of these fragments [Citation14]. Therefore, RA is generally not recommended in patients with poor ejection fraction (EF), and limited data are currently available concerning the in-hospital outcomes of PCI with RA in patients with low left ventricular EF (LVEF). Therefore, in the present study, we evaluated the angiographic success and in-hospital outcomes of PCI with RA in patients with low LVEF.
Methods
Study population ()
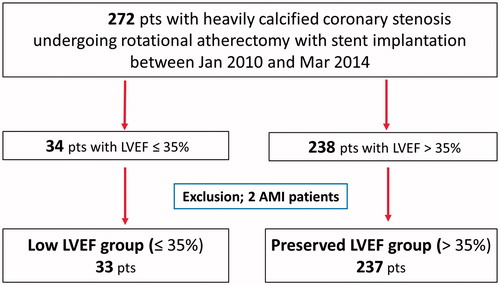
We retrospectively identified 272 consecutive patients who underwent RA followed by stent implantation for the treatment of heavily calcified coronary stenosis at New Tokyo Hospital between January 2010 and March 2014. Two patients with acute myocardial infarction (MI) were excluded from this study. A total of 270 patients were enrolled in this study. Of these, 33 patients had LVEF ≤35% (low LVEF group), and 237 patients had LVEF >35% (preserved LVEF group). Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. The EuroSCORE was calculated as previously described [Citation15,Citation16]. Informed consent was obtained from all individual participants included in the study.
Figure 1. Study flow chart. Two hundred seventy-two consecutive patients with heavily calcified coronary stenosis treated with RA followed by stent implantation at New Tokyo Hospital between January 2010 and March 2014 were retrospectively identified. Two patients with acute myocardial infarction were excluded from this study. Therefore, 270 patients were enrolled in this study. Of these, 33 patients had LVEF ≤35% (low LVEF group), and 237 patients had LVEF >35% (preserved LVEF group). RA: rotational atherectomy; LVEF: left ventricular ejection fraction; AMI: acute myocardial infarction.
Procedural details
The arterial access site was chosen according to the operator preference and anatomical availability. After cannulating the right or left coronary artery with a guiding catheter, a 0.009-inch Rotawire (Boston Scientific, Maple Grove, MN, USA) was directly advanced to the distal third of the coronary artery or following an exchange using a microcatheter or coaxial balloon. RA was performed using the Rotalink Plus system (Boston Scientific, Maple Grove, MN, USA). RA was performed using the Rotalink Plus system (Boston Scientific, Maple Grove, MN, USA). Ablations were performed at 210,000 to 230,000 rpm. A burr (range 1.25 to 2.25 mm) was used to treat the lesion. The burr size could be subsequently increased, or adjunctive coronary angioplasty could be performed at the operator’s discretion. Continuous intracoronary infusion of verapamil, nitro-glycerine and unfractionated heparin and pausing rotablation were performed to avoid slow flow. Care was taken to avoid a decrease of greater than 5,000 rpm in rotation speed. Temporary pacing was mandatory during RA. After successful atherectomy and consequent lesion modification, all patients received either bare metal stent (BMS) or DES implantation. All interventions were performed by experienced operators.
The stents used in this study were as follows: BMS; a paclitaxel-eluting stent (PES; Taxus; Boston Scientific, Natick, MA, USA); an endeavor zotarolimus-eluting stent (E-ZES; Endeavor, Endeavor Sprint; Medtronic CardioVascular Ltd, Santa Rosa, CA, USA); an everolimus-eluting stent (EES; Xience, Xience Prime and Xience Xpedition; Abbott Vascular, Japan, and Promus and Promus Element; Boston Scientific, Natick, MA, USA) and a resolute zotarolimus-eluting stent (R-ZES; Resolute Integrity; Medtronic CardioVascular Ltd, Santa Rosa, CA, USA).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Administered medication
All patients were treated before the procedure with aspirin (200 mg oral loading dose followed by 100 mg orally indefinitely) and an ADP receptor blocker, most commonly clopidogrel (300 mg oral loading dose followed by 75 mg orally for at least 12 months), ticlopidine (200 mg orally for at least 12 months) and prasugrel (3.75 mg orally for at least 12 months). Immediately before intervention, intra-arterial or intravenous heparin was administered to maintain an activated clotting time of ≥250 s. If the slow-flow phenomenon occurred, an intracoronary infusion of sodium nitroprusside and/or an intravenous infusion of noradrenalin was iteratively administered to improve coronary flow and maintain hemodynamic stability.
Study endpoints
The primary endpoint was angiographic success and in-hospital major advertise cardiac events (MACE). Angiographic success was defined as residual stenosis of <30% after stent implantation along with thrombolysis in myocardial infarct flow grade III at the end of the procedure. MACE was defined as the composites of death from any cause, post procedure onset MI, emergency coronary artery bypass grafting, and target vessel revascularization, and the components of MACE. The secondary endpoints were MACE and the components within 30days after PCI. The components of MACE were evaluated. Death was considered as cardiac in origin, unless obvious non-cardiac causes were identified. New-onset MI in elective cases with negative preintervention biomarkers was defined as creatine kinase muscle-brain type elevation greater than three-fold the upper normal level with Q waves or without Q waves on the electrocardiogram 24 hours after surgery.
Statistical analysis
Values were reported as the mean ± standard deviation. Differences in the categorical variables between the two groups were analysed with the χ2 test. Continuous variables were compared using the unpaired t-test or the non-parametric Wilcoxon rank sum test. All p-values were two-sided, and p < .05 was considered statistically significant. All analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics
The baseline clinical characteristics of the study population are presented in . In both groups, a high prevalence of DM, HT, and renal dysfunction was noted. The prevalence of previous MI and renal dysfunction were significantly increased in the low LVEF group compared with the preserved LVEF group. Furthermore, the low EF group included more hemodialysis (HD) patients compared with the preserved LVEF group. Logistic EuroSCORE was high in both groups, but the mean logistic EuroSCORE was significantly increased in the low LVEF group compared with the preserved LVEF group (13.8 vs. 5.54, p < .001).
Table 1. Baseline clinical characteristics.
Angiographic and procedural characteristics
Angiographic and procedural characteristics are presented in . Regarding the target vessel, left anterior descending artery (LAD) was less frequently involved in the low LVEF group compared with the preserved LVEF group (36.4% vs. 64.1%, p = .004). Furthermore, in our study, patients with left main disease were included (30.3% vs. 26.6%, p = .68). The use of intra-aortic ballon pumping (IABP) was significantly more frequent in the low LVEF group compared with the preserved LVEF group (15.2% vs. 2.1%, p = .003). In this study, IABP was used pre- procedure in eight patients. IABP was introduced mid-procedure in two patients owing to life-threatening hypotension during procedure. Implanted stent type was similar between two groups except TAXUS. Stent diameter and length did not significantly differ between both groups.
Table 2. Procedural characteristics.
Procedural and angiographic data
Procedural and angiographic data are presented in . All patients underwent RA and stent implantation. Two patients in the preserved EF group achieved only TIMI 2. The angiographic success rate was not significantly different between the two groups (100.0% vs 99.2%) p = 1.00. Significantly less contrast was used in the low LVEF group compared with the preserved LVEF group (123.5 cc vs. 149.6 cc p = .02). The complication rate during the procedure did not differ significantly between the two groups except slow flow. Slow flow occurred more frequently in the low LVEF group than in the preserved LVEF group (18.2% vs. 2.6%) (p = .001). However, TIMI 3 flow grade was obtained in all patients finally. The amount of administered nitroprusside and noradrenalin during the procedure was similar between the two groups.
Table 3. Procedural and angiographic data.
In hospital outcomes
In hospital outcomes were shown in . MACE rate was similar between both groups (3.0% in the low LVEF group vs. 1.3% in the preserved LVEF group, p = .41). Other in hospital outcomes were also significantly different between both groups.
Table 4. In hospital outcomes.
Clinical outcomes within 30 days
There were no significant differences between the two groups regarding clinical outcomes within 30 days following PCI ().
Table 5. Clinical outcomes within 30 days.
Discussion
A previous study reported that the angiographic success rate of PCI with RA in patients with preserved LVEF was high and that the incidence of peri-procedural complications, such as slow flow and postprocedural MI, was relatively low compared with patients with low LVEF [Citation17]. On the other hand, low LVEF has been reported to be an independent clinical predictor of long-term mortality [Citation18]. However, limited data are available on the angiographic success and in-hospital outcomes of PCI with RA in patients with low LVEF.
In our study, angiographic success was achieved in all cases. In addition, there were no significant differences in the in-hospital outcomes of PCI with RA between both groups.
Angiographic success and in-hospital outcomes
Severely calcified coronary lesions are classified as more complex ACC/AHA B2/C lesions requiring longer stents than non-calcified or mildly calcified lesions [Citation19–21]. Despite advances in interventional devices and techniques, the treatment of severely calcified coronary lesions remains challenging because these lesions respond poorly to balloon angioplasty and are associated with a high rate of restenosis [Citation22]. These lesions are also known to achieve lower success rates and higher complication rates following procedures, compared with non-calcified or mildly calcified lesions [Citation20,Citation21]. RA is used to treat severely calcified and otherwise non-dilatable or non-crossable lesions [Citation14]. Furthermore, other previous studies have reported that decreased LVEF is an independent risk factor for in-hospital mortality following conventional PCI and RA stenting [Citation18,Citation23]. For the above-mentioned reasons, RA stenting is typically performed for patients with preserved LVEF in actual clinical practice. However, limited data are currently available on the outcomes of PCI with RA in patients with low LVEF.
Then, our study evaluated the angiographic success rate and in-hospital MACE of PCI with RA in patients with low LVEF. The outcomes of patients with low LVEF were similar to those of patients with preserved LVEF in this study. In this study, less LAD lesions were treated in low LVEF group. However, we considered that there were no impact on the result because the rate of in hospital MACE did not significantly differ between patients treated for LAD lesion and non LAD lesions (1.8% vs. 0.9%, p = 1.00). Also, the rate of MACE within 30 days after PCI did not significantly differ between those patients (3.7% vs. 1.9%, p = .49).
Some patients manifested with a slow flow phenomenon and developed life-threatening hypotension during the procedure. In such cases, noradrenaline was administered to increase blood pressure regardless of low or preserved LVEF. In some cases, nitroprusside was also administered to improve the slow flow phenomenon regardless of low or preserved LVEF. If medications were insufficient to ensure hemodynamic stability, we performed mechanical hemodynamic support by IABP. Interestingly, the amount of administered noradrenalin and nitroprusside were not significantly different between both groups. On the other hand, the use of IABP was significantly more frequent in the low LVEF group compared with the preserved LVEF group. Nevertheless, angiographic success was achieved in all cases, and no significant differences in the in-hospital MACE of PCI with RA were noted between both groups in our study.
To the best of our knowledge, if medications and mechanical support were appropriately performed, the angiographic success rate and in-hospital MACE rate of PCI with RA in patients with low LVEF could be expected to have good outcomes similar to those in patients with preserved LVEF. This study provides novel evidence about PCI with RA in patients with low LVEF.
Study limitations
The main limitation of this study was the lack of randomization and the retrospective, observational design. Furthermore, the size of the study population was limited. Further trials with a prospective design, a larger cohort of patients, and a longer follow-up are warranted.
Conclusions
If medications and mechanical support were appropriately performed, the angiographic success rate and in-hospital MACE rate of PCI with RA in patients with low LVEF could be expected to have good outcomes similar to those in patients with preserved LVEF.
Disclosures statement
No potential conflict of interest was reported by the author(s).
References
- Brodie BR, Stuckey T, Downey W, et al. Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. JACC Cardiovasc Interv. 2008;1:405–414.
- Otsuka M, Toyofuku M, Watanabe N, et al. Clinical usefulness of drug-eluting stents in the treatment of dialysis patients with coronary artery disease. EuroIntervention. 2011;6:754–759.
- Smits PC, Kedhi E, Royaards KJ, et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). J Am Coll Cardiol. 2011;58:11–18.
- Raber L, Juni P, Nuesch E, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol. 2011;57:2143–2151.
- Taniwaki M, Stefanini GG, Silber S, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2014;63:1617–1625.
- Fujimoto H, Nakamura M, Yokoi H. Impact of calcification on the long-term outcomes of sirolimus-eluting stent implantation: subanalysis of the Cypher Post-Marketing Surveillance Registry. Circ J. 2012;76:57–64.
- Fujii K, Mintz GS, Kobayashi Y, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109: 1085–1088.
- Hong MK, Mintz GS, Lee CW, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–1310.
- Stertzer SH, Rosenblum J, Shaw RE, et al. Coronary rotational ablation: initial experience in 302 procedures. J Am Coll Cardiol. 1993;21:287–295.
- Warth DC, Leon MB, O'Neill W, et al. Rotational atherectomy multicenter registry: acute results, complications and 6-month angiographic follow-up in 709 patients. J Am Coll Cardiol. 1994;24:641–648.
- Reisman M, Harms V, Whitlow P, et al. Comparison of early and recent results with rotational atherectomy. J Am Coll Cardiol. 1997;29:353–357.
- Ahn SS, Auth D, Marcus DR, et al. Removal of focal atheromatous lesions by angioscopically guided high-speed rotary atherectomy. Preliminary experimental observations. J Vasc Surg. 1988;7:292–300.
- Ritchie JL, Hansen DD, Intlekofer MJ, et al. Rotational approaches to atherectomy and thrombectomy. Zeitschrift Fur Kardiol. 1987;76(Suppl 6):59–65.
- Couper LT, Loane P, Andrianopoulos N, et al. Utility of rotational atherectomy and outcomes over an eight-year period. Catheter Cardiovasc Interv. 2015.
- Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13.
- Romagnoli E, Burzotta F, Trani C, et al. EuroSCORE as predictor of in-hospital mortality after percutaneous coronary intervention. Heart (British Cardiac Society). 2009;95:43–48.
- Jinnouchi H, Kuramitsu S, Shinozaki T, et al. Two-year clinical outcomes of newer-generation drug-eluting stent implantation following rotational atherectomy for heavily calcified lesions. Circ J. 2015;79:1938–1943.
- Edes IF, Ruzsa Z, Szabo G, et al. Clinical predictors of mortality following rotational atherectomy and stent implantation in high-risk patients: A single center experience. Cathet Cardiovasc Intervent. 2015;86:634–641.
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510–518.
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 1992;86:64–70.
- Gilutz H, Weinstein JM, Ilia R. Repeated balloon rupture during coronary stenting due to a calcified lesion: an intravascular ultrasound study. Cathet Cardiovasc Intervent. 2000;50:212–214.
- Onuma Y, Tanimoto S, Ruygrok P, et al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: two-year angiographic and three-year clinical results from the SPIRIT II study. Cathet Cardiovasc Intervent. 2010;76:634–642.
- Wu C, Hannan EL, Walford G, et al. A risk score to predict in-hospital mortality for percutaneous coronary interventions. J Am Coll Cardiol. 2006;47:654–660.
