Abstract
Objective. We aim to determine the correlation between ST-segment changes in leads V4–V6 and the extent of myocardial injury by cardiac magnetic resonance (CMR) in patients with inferior ST elevation (STE) myocardial infarction (iSTEMI). Design. Admission electrocardiogram and CMR data from the MITOCARE trial were used. Differences in mean myocardium at risk, infarct size, ejection fraction and myocardial segment involvement by CMR were compared in patients with first iSTEMI with STE, ST depression (STD) or no ST changes (NST) in V4–V6. Myocardial segment involvement was further evaluated by comparing proportion of patients in each group with ≥25% and ≥50% segment involvement. Results. Fifty-four patients were included. Patients with STE (n = 22) and STD (n = 16) in V4–V6 had significantly lower ejection fraction compared to NST (n = 16) (48% vs 48% vs 54%, p = .02). STE showed more apical, apical lateral and mid-inferolateral involvement but less basal inferior involvement than NST. STD exhibited greater basal inferoseptal involvement compared to STE. There were more patients with STE that had ≥25% and ≥50% apical lateral involvement compared with STD and NST groups. Patients with STD were more likely to have ≥25% and ≥50% basal inferoseptal involvement compared with STE and NST groups.Conclusion. Our study suggests that in iSTEMI, ST changes in the precordial leads V4–V6 correlates with greater myocardial injury and distribution of myocardium at risk.
Introduction
The electrocardiogram (ECG) remains an essential tool for the evaluation of patients with acute myocardial infarction. In the setting of an ST elevation (STE) myocardial infarction (STEMI), the ECG can quickly provide clinicians with valuable information on the presence, severity and extent of cardiac injury. In acute inferior STEMI (iSTEMI), ECG typically demonstrates STE in leads II, III and aVF. Several studies have shown that ST depression (STD) in the precordial leads V4–V6 are associated with worse outcomes than those without these precordial ST changes [Citation1–3]. STD in V4–V6 have been correlated with worse left ventricular (LV) dysfunction as well as concomitant left anterior descending artery stenosis or triple vessel disease [Citation4–6]. STE in V5 has been linked with more frequent involvement of the apical portion of the inferior wall whereas STE in V6 was associated with involvement of the mid-posterior segment on echocardiogram [Citation7,Citation8]. STE in leads V5–V6 have been associated with involvement of a large posterolateral branch of the right coronary artery or a large marginal branch of the left circumflex artery [Citation7].
Thus far, most studies correlating ECG and the location of myocardial segment ischemia have largely been extrapolated from indirect information – i.e. regional wall motion abnormalities assessed by echocardiography, perfusion defects from scintigraphy and angiographic data. Cardiac magnetic resonance imaging (CMR) provides a powerful means to directly examine areas of myocardial injury [Citation9]. Late Gadolinium Enhancement (LGE) CMR provides an accurate method for measuring myocardial infarct distribution and size [Citation10]. Moreover, contrast enhanced steady-state-free precession (CE-SSFP) have been shown to be effective in determining the myocardium at risk up to 1 week after the acute event [Citation11].
Some previous studies have used CMR to examine the extent of myocardial damage in patients with T-wave inversions and pathological Q-waves after acute myocardial infarction (AMI) [Citation12,Citation13]. However, there is no CMR studies that examines cardiac segment involvement in patients with iSTEMI and precordial ST-changes in V4–V6. The aim of this study is to examine how patterns of precordial ST-changes in leads V4–V6 correlate with myocardial segment injury seen on CMR in patients presenting with iSTEMI.
Methods
Patients
We analyzed patients enrolled in the MITOCARE study, a phase II, multicenter, randomized, double blind, placebo-controlled study, which examined the effect of the drug TRO40303 in patients presenting with first-time STEMI and treated with primary percutaneous coronary intervention (PCI) [Citation14,Citation15]. All patients in the MITOCARE study were at least 18 years old with a first-time STEMI (defined as nitrate resistant chest pain ≥30 min, and new STE at J-point in two contiguous leads with cut-off points: ≥0.2 mV in men or ≥0.15 mV in women in leads V2–V3 and/or ≥0.1 mV in other leads). All patients in the study presented within 6h of the onset of chest pain. Exclusion criteria included previous myocardial infarction, previous coronary artery bypass surgery, and known ischemic cardiomyopathy [Citation15].
CMR imaging
In this study, CMR was used to measure myocardium at risk and infarct size between the test drug and placebo, and showed no difference between the two. CMR images were acquired 3–5 days after STEMI. Myocardium at risk and left ventricular function were assessed by CE-SSFP imaging [Citation11]. Infarct size was assessed by LGE according to previously described methodology [Citation16]. The detailed parameters of the CMR protocol is described in the original MITOCARE studies.
Electrocardiographic criteria
Patients with both available CMR data and admission ECG with evidence of iSTEMI (defined as STE ≥0.1 mV in at least two of three inferior leads II, III, aVF) were included. Patients with ventricular rhythms, ventricular paced rhythm and those with incomplete admission ECG data were excluded. We also excluded patients with both anterior (leads V1–V3) and inferior STE, where the summation of the anterior STE exceeded that of the inferior STE (more likely representing anterior STEMI). Patients were categorized based on V4–V6 precordial ST changes as either elevation, depression or no ST changes (NST). STE and STD were defined as change of ≥0.1 mV in at least one of the V4–V6 leads.
Statistical analysis
Baseline characteristics were obtained for each patient. Categorical data between each group was compared using chi-squared test or fisher’s exact test depending on sample size, while continuous data was compared by one-way analysis of variance (ANOVA). To evaluate how precordial ST changes correlated with areas of ischemia based on magnetic resonance, we collected data on myocardium at risk of the overall left ventricle (LV) as well as involvement of each LV segment based on the 17-segment model [Citation17]. Information on overall infarct size and ejection fraction (EF) was also obtained for each patient. Differences in overall myocardium at risk, infarct size and EF were compared using one-way ANOVA followed by Tukey’s post hoc analysis for significant differences. We approached the assessment of LV segment involvement in two ways. First, we compared the mean segment involvement for each group via ANOVA with post-hoc test. Second, we tabulated the number of patients in each group with ≥25% segment involvement and repeated this process for ≥50% involvement. We then compared the number of individuals with ‘significant’ segment involvements using chi-square test. ANOVA and post hoc tests were performed using R statistical software. Chi-square tests were performed using Microsoft Excel.
Results
Baseline characteristics
In the MITOCARE study, 98 of the 163 patients enrolled had iSTEMI. Of these, 54 patients had complete, high-quality CMR studies and were included in the study – 16 patients had STD, 22 had STE and 16 had NST in leads V4–V6. Ten of the 16 patients with NST in V4–V6, had no ST changes in all precordial leads (V1–V6). CMR imaging examples from each ST-segment group is shown in . Most of the patient had RCA as the culprit artery, with no difference among the three groups. Patients with STE had significantly less post-PCI TIMI 0–1 flow as well as higher mean presenting troponin when compared to patients with STD or NST. Otherwise, baseline characteristics including age, gender, hypertension, diabetes, hyperlipidemia, smoking, pre-PCI TIMI flow and symptom to balloon time were similar among groups ().
Figure 1. Representative CMR images of the three groups. Depicted sectors with asterixis indicate myocardium at risk. Top column shows case with ST depression, middle row no ST change, and bottom row, with ST elevation. Left column represents a basal slice, middle column mid ventricular slice, and right column apical slice.
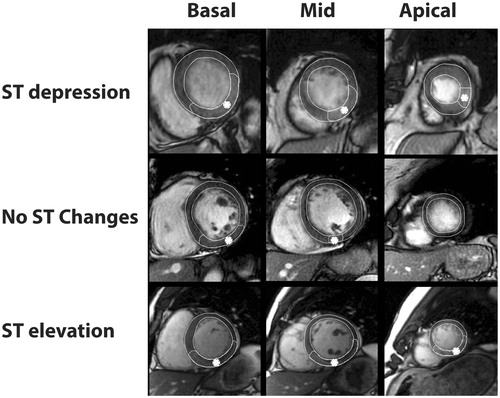
Table 1: Baseline characteristics.
Table 2. Overall myocardium at risk, infarct size and ejection fraction.
Overall myocardial injury ()
The mean myocardium at risk based on percent LV involvement was larger in the STE and STD group compared with the NST group with results approaching but not achieving statistical significance (p = .08). Likewise, iSTEMIs with STE and STD in V4–V6 tended to have larger average infarct size compared to NST though results did not reach statistical significance (p = .10). ISTEMIs with STE in V4–V6 had significantly lower EF compared with those with NST in those leads (48% to 54%, p = .03 on post hoc). ISTEMIs with STD in V4–V6 also appeared to have lower EF compared to NST, though p-value approached but did not achieve significance (48% to 54%, p = .06 on post hoc).
Segmental involvement
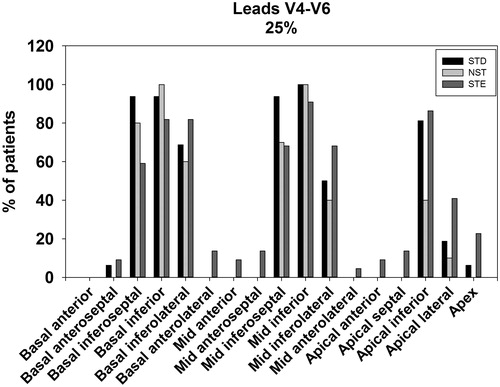
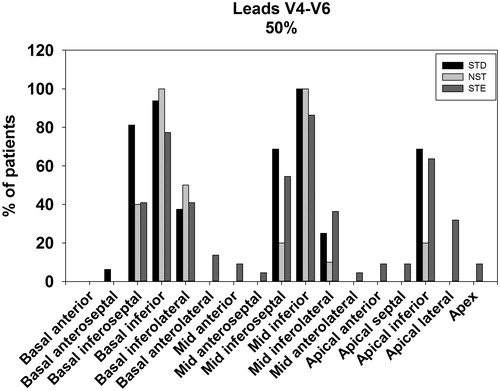
Patients with STE in V4–V6 tended towards having more myocardium at risk in the apical (p = .03 on post hoc), apical lateral (p = .006 on post hoc) and mid-inferolateral segments (p = .037 on post hoc) when compared with patients with NST in V4–V6. In contrast, STE V4–V6 were associated with less basal inferior involvement compared to the NST group (p = .01 on post hoc). Overall, the pattern of myocardium at risk distribution in the STE group tended towards more involvement of the apical and lateral segments (). This pattern was also observed when assessing the proportion of patients with ≥25% and ≥50% myocardium at risk in each segment ( and ). On chi-square comparison, there were significantly more patients with STE that had ≥25% apical lateral segment involvement compared with the STD and NST groups (41% vs. 19% vs. 6%, p = .04). This was also observed for ≥50% segmental involvement (STE 32% vs. STD 0% vs. NST 0%, p = .003).
Table 3. Mean myocardium at risk per segment.
STD in V4–V6 exhibited greater mean basal inferoseptal involvement compared with STE (p = .0004 on post hoc), but not significantly different when compared to NST (p = .08). Overall, distribution of myocardium at risk tended towards more extension to the inferoseptal regions (). This corroborated with results comparing the number of individuals in each group with ≥25% or ≥50% myocardium at risk per myocardial segment ( and ). On chi-square comparison, patients with STD were more likely to have ≥25% involvement in the basal inferoseptal segment compared with STE and NST groups (94% vs. 59% vs. 88%, p = .02). This was again observed for ≥50% segmental involvement for the basal inferoseptal segment (STD 81% vs. STE 41% vs. NST 38%, p = .02).
Discussion
Precordial ST changes and extent of myocardial injury
Our study suggests that in iSTEMI, ST changes in the precordial leads V4–V6, especially STE, correlated with greater myocardial injury. Patients with either STE or STD had lower ejection fraction compared to patients without ST changes in these leads. Furthermore, both the mean myocardium at risk as well as infarct size tended to be bigger in iSTEMIs with ST changes, though results did not reach significance. Historically, decrease in left ventricular ejection fraction (LVEF) is an important prognostic indicator for survival after AMI [Citation18]. More recent studies examining the prognostic impact of reduced CMR-derived ejection fraction have shown LVEF to be an independent predictor of major adverse cardiovascular events (MACE) after AMI [Citation19–22]. A meta-analysis on the prognostic value of CMR findings for future cardiovascular events in patients with AMI found LVEF to be the only predictor for MACE with a hazard ratio of 1.03 to 1.05 per % decrease in LVEF [Citation23]. Though we did not have outcomes data and could not directly correlate ECG changes, LVEF and survival, our results suggest that previous findings of worse in-hospital mortality [Citation1,Citation2] and one-year mortality [Citation4] in iSTEMI associated with ST changes in V4–V6 may stem in part from more depressed LVEF. ST changes in V4–V6 in iSTEMI, therefore, likely represent a high-risk feature that should prompt expedient revascularization and close follow up care in an effort to minimize the extend of myocardial injury [Citation24].
The differences in LV dysfunction as well as myocardium at risk and infarct size seen on CMR were most striking in the STE group. Assali et. al. previously demonstrated that STE in V5–V6 is correlated with MIs associated with large posterolateral branches of either the right coronary artery or left circumflex artery and suggested that this subgroup of iSTEMIs were associated with larger perfusion territory [Citation7]. Meanwhile, other studies have shown that iSTEMIs with STE in leads V4-V6 were associated with larger infarct size [Citation8,Citation25]. Our study supports previous findings that suggest STE in the lateral precordial leads serve as a marker for higher ischemic burden. However, it should be noted that 25% revascularization attempts of the STD and NST groups resulted in only TIMI 0–1 flow, while all STE patients achieved post-PCI TIMI 2–3 flow. This difference may have had an influence on the areas of ischemia found in the respective groups.
Data on area of myocardial injury in patients with STD in leads V4–V6 are limited, though Birnbaum et. al. showed in a previous study that infarct size, as estimated by level of serum creatinine kinase, was similar between STD and no ST changes [Citation4]. Our results are equivocal when compared to previous findings as we demonstrate that area of ischemia, size of infarct as well as troponin levels are all modestly increased in the STD group compared to the NST group, but the differences did not reach statistical significance. STD in V4–V6 in iSTEMIs has previously been shown to be associated with left anterior descending artery (LAD) involvement and multi-vessel vessel disease [Citation4–6]. The increased prevalence of multi-vessel involvement presents a possible explanation for the greater extent of LV dysfunction and more involvement of the inferoseptal segments (due to lack of collateral flow from the LAD) in this group.
Precordial ST changes in regional myocardial involvement
Our study demonstrates differences in the distribution of myocardium at risk with respect to ST deviation in leads V4–V6. STE in leads V4–V6 was associated with a more lateral and apical extension, as well as a tendency towards less basal inferior and inferoseptal involvement. Thus, in iSTEMIs, STE in the lateral precordial leads reflect true extension of ischemia to the lateral segments.
Three previous angiography studies suggest that STE and Q-waves in the lateral precordial leads in the setting of iSTEMI are suggestive of a culprit LCx artery [Citation26–28]. However, other studies did not observe this association [Citation7,Citation29]. Our results too showed very few culprit LCx lesions (). Warner et. al. even postulated that Q-waves in V5–V6 may represent more apical infarcts based on more apical akinesia but not lateral wall ischemia, as they did not find significant disease in the arteries that traditionally supply the lateral wall [Citation29]. We found that both apical and lateral segment extension occur in the group with STE in V5–V6, and that lateral ischemia may be present in the absence of primary LCx lesions. It is possible that the more lateral and apical ischemia can be attributed to involvement of prominent posterolateral branches, as proposed in Assali et. al, though further angiographic evidence is needed to verify this point [Citation7].
ISTEMIs with STD in V4–V6 were associated with a more septal involvement but no appreciable increase in lateral wall ischemia. Based on these findings, STD in the lateral precordial leads may represent to some degree reciprocal changes from an opposing ischemic zone, rather than subendocardial ischemia. STD in V4–V6, as discussed in the above section, has been shown to correlate with more LAD involvement [Citation5,Citation6]. Studies on vascular territories demonstrate that branches off the LAD often provide secondary blood supply to the inferoseptal segments [Citation30]. Thus, the increased prevalence of LAD involvement may account for the increase in ischemia in this region as observed in our study. We did not find more anterior involvement in these patients. However, the ability of CMR to detect subendocardial ischemia secondary to demand supply mismatch has not been validated and it might be that the CMR is not sensitive for concomitant anterior subendocardial ischemia.
Limitations
There were several limitations to our study. First, our overall sample size was small, which dampens the strength of our results. Secondly, the number of patients with purely lateral precordial ST changes and isoelectric ST segments in V1–V3 was limited. Because of this, many of our patients had some degree of V1–V3 ST changes on the ECG. Thirdly, while ECG’s were obtained at admission, CMR was obtained 3 to 5 days afterwards. Given this temporal difference, findings on initial ECG may not always reflect the final area of ischemic injury due to the oft dynamic evolution of AMI’s. Lastly, our study had limited angiographic data and thus we could not adequately assess relationships between ischemic regions and coronary vascular territories. The association between CMR and angiographic findings in iSTEMIs merits further investigation.
Conclusion
Cardiac magnetic resonance provides a unique perspective in the study of ECG changes during myocardial infarction, affording direct visualization of the extent and location of myocardial injury. Our findings suggest that in the setting of iSTEMI, precordial changes in leads V4–V6 is associated with lower ejection fraction, which represents a prognostic indicator for poorer outcomes. Furthermore, STE in V4–V6 is associated with a more apical and lateral distribution while STD is associated with a more septal distribution.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Hasdai D, Sclarovsky S, Solodky A, et al. Prognostic significance of maximal precordial ST-segment depression in right (V1 to V3) versus left (V4 to V6) leads in patients with inferior wall acute myocardial infarction. Am J Cardiol. 1994;74:1081–1084.
- Peterson ED, Hathaway WR, Zabel KM, et al. Prognostic significance of precordial ST segment depression during inferior myocardial infarction in the thrombolytic era: results in 16,521 patients. J Am Coll Cardiol. 1996;28:305–312.
- Hlatky MA, Califf RM, Lee KL, et al. Prognostic significance of precordial ST-segment depression during inferior acute myocardial infarction. Am J Cardiol. 1985;55:325–329.
- Birnbaum Y, Wagner GS, Barbash GI, et al. Correlation of angiographic findings and right (V1 to V3) versus left (V4 to V6) precordial ST-segment depression in inferior wall acute myocardial infarction. Am J Cardiol. 1999;83:143–148.
- Strasberg B, Pinchas A, Barbash GI, et al. Importance of reciprocal ST segment depression in leads V5 and V6 as an indicator of disease of the left anterior descending coronary artery in acute inferior wall myocardial infarction. Br Heart J. 1990;63:339–341.
- Hasdai D, Birnbaum Y, Porter A, et al. Maximal precordial ST-segment depression in leads V4–V6 in patients with inferior wall acute myocardial infarction indicates coronary artery disease involving the left anterior descending coronary artery system. Int J Cardiol. 1997;58:273–278.
- Assali AR, Sclarovsky S, Herz I, et al. Comparison of patients with inferior wall acute myocardial infarction with versus without ST-segment elevation in leads V5 and V6. Am J Cardiol. 1998;81:81–83.
- Tsuka Y, Sugiura T, Hatada K, et al. Clinical characteristics of ST-segment elevation in lead V6 in patients with Q-wave acute inferior wall myocardial infarction. Coron Artery Dis. 1999;10:465–469.
- Carlsson M, Ubachs JF, Hedström E, et al. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–576.
- West AM, Kramer CM. Cardiovascular magnetic resonance imaging of myocardial infarction, viability, and cardiomyopathies. Curr Probl Cardiol. 2010;35:176–220.
- Sörensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. J Cardiovasc Magn Reson. 2010;12:25.
- Delewi R, Ijff G, van de Hoef TP, et al. Pathological Q waves in myocardial infarction in patients treated by primary PCI. JACC Cardiovasc Imaging. 2013;6:324–331.
- Nijveldt R, van der Vleuten PA, Hirsch A, et al. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc Imaging. 2009;2:1187–1194.
- Atar D, Abitbol JL, Arheden H, et al. Rationale and design of the 'MITOCARE' Study: a phase II, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of TRO40303 for the reduction of reperfusion injury in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Cardiology. 2012;123:201–207.
- Atar D, Arheden H, Berdeaux A, et al. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. 2015;36:112–119.
- Heiberg E, Ugander M, Engblom H, et al. Automated quantification of myocardial infarction from MR images by accounting for partial volume effects: animal, phantom, and human study. Radiology. 2008;246:581–588.
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542.
- Norris RM, Barnaby PF, Brandt PW, et al. Prognosis after recovery from first acute myocardial infarction: determinants of reinfarction and sudden death. Am J Cardiol. 1984;53:408–413.
- Husser O, Monmeneu JV, Sanchis J, et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol. 2013;167:2047–2054.
- de Waha S, Desch S, Eitel I, et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–2668.
- Eitel I, Desch S, de Waha S, et al. Long-term prognostic value of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. Heart. 2011;97:2038–2045.
- Cochet AA, Lorgis L, Lalande A, et al. Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol. 2009;19:2117–2126.
- El Aidi H, Adams A, Moons KG, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease. A systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031–1045.
- Atar D, Agewall S. End of story? Studies on prevention of reperfusion injury encounter perpetual defeats. Eur Heart J Cardiovasc Pharmacother. 2015;1:46–47.
- Clemmensen P, Grande P, Aldrich HR, et al. Evaluation of formulas for estimating the final size of acute myocardial infarcts from quantitative ST-segment elevation on the initial standard 12-lead ECG. J Electrocardiol. 1991;24:77–83.
- Bairey CN, Shah PK, Lew AS, et al. Electrocardiographic differentiation of occlusion of the left circumflex versus the right coronary artery as a cause of inferior acute myocardial infarction. Am J Cardiol. 1987;60:456–459.
- Huey BL, Beller GA, Kaiser DL, et al. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol. 1988;12:1156–1166.
- Hiasa Y, Morimoto S, Wada T, et al. Differentiation between left circumflex and right coronary artery occlusions: studies on ST-segment deviation during percutaneous transluminal coronary angioplasty. Clin Cardiol. 1990;13:783–788.
- Warner RA, Hill NE, Mookherjee S, et al. Diagnostic significance for coronary artery disease of abnormal Q waves in the “lateral” electrocardiographic leads. Am J Cardiol. 1986;58:431–435.
- Cerci RJ, Arbab-Zadeh A, George RT, et al. Aligning coronary anatomy and myocardial perfusion territories: an algorithm for the CORE320 multicenter study. Circ Cardiovasc Imaging. 2012;5:587–595.