Abstract
Objective. Dabigatran is a direct thrombin inhibitor. As the main adverse event is bleeding, it is relevant whether dabigatran has additional effects on platelet function. If so, it could affect the bleeding risk. We aimed to assess in vitro aggregation in patients with atrial fibrillation (AF) receiving dabigatran. Design. We evaluated 32 AF patients treated with dabigatran (study group) and 18 non-anticoagulated non-AF blood donors (control group). We assessed light transmittance platelet aggregation (LTA) with 100 nmol/L γ-thrombin in both groups. The LTA was performed at two time-points in our dabigatran group of patients. Results. The thrombin-induced platelet aggregation was significantly lower two hours after dabigatran was taken compared to baseline measurement (9% ± 6% vs. 29% ± 21%) in our study group. Moreover, we observed that the baseline value of platelet aggregation in patients on dabigatran treatment was significantly lower compared to healthy volunteers (29% ± 21% vs. 89 ± 8). However, one subanalysis showed that this significant reduction in platelet aggregation at baseline was only observed in patients who received dabigatran for over a week. Conclusion. The thrombin-induced platelet aggregation is reduced in non-valvular AF patients receiving dabigatran after a week-long therapy.
Introduction
Dabigatran etexilate is a low-molecular-weight prodrug that exhibits no pharmacological activity. After oral administration, dabigatran etexilate is converted to its active form, dabigatran, a potent, competitive, and reversible direct inhibitor of thrombin [Citation1–5]. Dabigatran binds to the active site of the thrombin, thereby inactivating both fibrin-bound and unbound (free) thrombin [Citation6]. As dabigatran is a direct thrombin inhibitor, it has effect on the secondary haemostasis [Citation7]. As the main adverse event of dabigatran treatment is bleeding [Citation8], it is relevant whether dabigatran has additional effects on platelet function. If so, it could affect the bleeding risk and influence the choice of treatment based on the patient risk profile. The effect of dabigatran on platelet activation has been studied by Vinholt et al. [Citation9]. In this study healthy donor blood was incubated with dabigatran 0, 50, 500 and 10,000 ng/mL. It was observed that the platelet activation was reduced after thrombin stimulation in samples with dabigatran levels ≥500 ng/mL. It could be an indirect effect due to the binding of dabigatran to generated thrombin which prevents thrombin from stimulating platelets. Platelets express two thrombin receptors, protease-activated receptor (PAR)-1 and PAR-4, which are both stimulated with thrombin [Citation10]. The aim of the present study was to assess the effects of dabigatran on in vitro platelet aggregation in real patients receiving dabigatran.
Material and methods
The local Ethical Committee of the Jessenius Faculty of Medicine in Martin approved this study (EK 1702/2015). All study participants agreed to participate in the project and signed a written informed consent in accordance with the Declaration of Helsinki.
Dabigatran was administrated in patients with atrial fibrillation (AF) twice daily (at 7:00 PM and 7:00 AM). Blood samples were taken 12 hours after a previous drug dose administration (baseline, at 7:00 AM), followed by next blood sample after 2 hours (at 9:00 AM). To be sure that the drug was administered at the right and same time, we implemented the following measures. First, the drug was administered to the patients by a physician who was involved in this study. Second, treatment with dabigatran was monitored by Hemoclot Thrombin Inhibitor assay (Hyphen BioMed, Paris, France), according to the manufacturer’s instructions. This dilute thrombin time assay is certified in Europe and the United States for the quantitative determination of dabigatran plasma levels. All patients on dabigatran therapy included in our study where hospitalized on the 1st Department of Internal Medicine during July and August 2017. We did not have any selection criteria. Concomitant treatment (e.g. beta blockers, proton-pump inhibitor…) was administered immediately after taking the morning dose of dabigatran.
We recruited 18 blood donors aged 19 to 61 years for the control group. These patients were healthy volunteers without any current drug history. Blood samples were collected from each donor and thrombin platelet aggregation was measured.
Light transmission aggregometry (LTA) was performed using the international protocol for the laboratory investigation of platelet function [Citation11]. We want to emphasize that testing was performed on patients without any antiplatelet or non-steroidal anti-inflammatory drugs (10–14 days before measurement) and with normal platelet count (≥150 × 109/L). The antecubital venous blood was collected into tubes containing 3.2% buffered sodium citrate (anticoagulant-blood ratio 1:9) to assess platelet aggregation. Platelet aggregability was tested with platelet-rich plasma (PRP) using platelet aggregometry (PACKS-4 aggregometer, Helena Laboratories, USA). The platelet count in PRP was between 400–450 × 109/L. Blood samples were stimulated with human γ-thrombin in final concentration 100 nmol/L (Mybiosource Inc., San Diego, USA).
Data are presented as numbers with frequencies for categorical variables and means with standard deviations (± SD) for continuous variables. For comparison of the different groups, the closed-test-principle was used. An overall comparison was performed, followed by pairwise comparison if the results were significant. The p values less than 0.05 were considered statistically significant. Data were analysed with SPSS 21.0.0.0 (SPSS Inc, Chicago, Illinois, USA).
Results
presents full clinical baseline characteristics of the patients. Thirty-two patients with non-valvular AF were enrolled (study group). The mean age was 72 ± 9 years (range 51-88 years), 14 patients were woman and the mean CHA2DS2VASc score was 3 ± 1. All patients began treatment with dabigatran as an initial anticoagulant treatment. Eight patients had an initial duration ≤7 days. Dabigatran doses were 110 mg (59%) or 150 mg (41%) twice daily.
Table 1. Clinical baseline characteristics of the patients.
In sum, data from 18 blood donors were included in the analyses (control group). The mean age was 35 ± 11 years (range 19-61 years), 7 blood donors were woman.
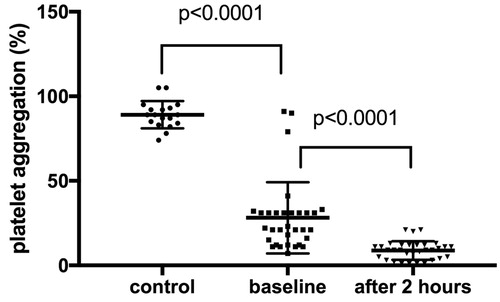
As shown in , the thrombin-induced platelet aggregation was significantly lower two hours after dabigatran was taken compared to baseline measurement (9% ± 6% vs. 29% ± 21%; p < .0001) in our study group. Moreover, we observed that the baseline value of platelet aggregation in patients on dabigatran treatment was significantly lower compared to healthy volunteers (29% ± 21% vs. 89 ± 8; p < .0001).
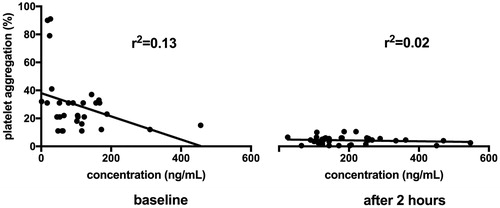
The mean dabigatran concentration at baseline was 104 ± 91 ng/mL and 190 ± 111 ng/mL two hours after dabigatran was taken, respectively. The dose-response curve for the plasma-diluted thrombin time assay with dabigatran had a correlation coefficient of r2 = 0.13 for baseline and 0.02 for followed measurement (after two hours), see .
Figure 2. The dose-response curve for the plasma-diluted thrombin time assay with dabigatran. Line represent the best-fit regressions for dabigatran.
We have done 17 subgroup analyses in order to determine the impact on aggregometer measurement, see . We did not find any significant difference between the groups, with one exception. Thrombin induced platelet aggregation was depended on treatment duration. In short, we did not observe significant difference between healthy volunteers and baseline aggregation values for patients receiving dabigatran <7 days (89 ± 8 vs. 73% ± 24%; p > .05). Moreover, we observed significant difference between patients receiving dabigatran <7 days vs. >7 days (baseline measurement: 73% ± 24% vs. 28% ± 20%, p = .02; measurement after two hours: 25% ± 7% vs. 8% ± 5%, p = .03).
Table 2. Subgroup analysis
Discussion
Dabigatran is a competitive reversible non-peptide antagonist of thrombin. Thrombin is not only a key protein in the cascade of fibrin clot formation but also a potent inducer of platelet aggregation [Citation12–17]. This is the first prospective comprehensive study to test whether the dabigatran affects thrombin induced platelet aggregation.
This case-control study quantifies platelet aggregation in patients treated with dabigatran by LTA. The thrombin-induced platelet aggregation was significantly lower two hours after dabigatran was taken compared to baseline measurement in our study group. Moreover, we observed that the baseline value of platelet aggregation in patients on dabigatran treatment was significantly lower compared to healthy volunteers. However, we found a significant difference between patients receiving dabigatran <7 days vs. >7 days. In addition, we did not observe significant difference between healthy volunteers and baseline aggregation values for patients receiving dabigatran <7 days. This indicates that the reduction of thrombin inducted platelet aggregation is not necessarily an immediate effect of dabigatran but may be explained by the duration of chronic dabigatran therapy. Similar results were observed by TRAP-induced platelet aggregation in patients on dabigatran or xabans treatment [Citation18,Citation19]. Thus, data from our study and study which dealt with TRAP-induced platelet aggregation may indicate that aggregation is enhanced by a chronic, direct and indirect inhibition of thrombin. This might be due to changes in expression of PAR-1 and PAR-4 receptor on platelets.
No significant differences were found in the patients with a history of renal or liver disease, diabetes, coronary artery disease, myocardial infarction, and so on (see ).
Our findings could have some important clinical implications because platelet aggregation and coagulation cascade are affected at the same time. The situation could be more worse during concomitant administration of antiplatelet or anticoagulant agents.
There were several limitations in our study. The small number of participants may have limited the ability to detect small drug effects on platelet function. Second, this study was not powered for clinical outcome. Therefore, it cannot be concluded that, for example, combination of antiplatelet therapy and dabigatran is not safe. Third, platelet aggregability is greatly affected by preanalytical issues and therefore interpretation of platelet hyperaggregability is potentially accordingly adversely influenced.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1–22.
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353(10):1028–1040.
- Wändell P, Carlsson AC, Sundquist J, Johansson SE, Bottai M, Sundquist K. Effect of cardiovascular drugs on mortality in atrial fibrillation and chronic heart failure. Scand Cardiovasc J. 2014;48(5):291–298.
- Maaroos M, Pohjantähti-Maaroos H, Halonen J, Vähämetsä J, Turtiainen J, Rantonen J, Hakala T, Mennander AA, Hartikainen J. New onset postoperative atrial fibrillation and early anticoagulation after cardiac surgery. Scand Cardiovasc J. 2017;51(6):323–326.
- Wändell P, Carlsson AC, Holzmann MJ, Ärnlöv J, Johansson SE, Sundquist J, Sundquist K. Warfarin treatment and risk of stroke among primary care patients with atrial fibrillation. Scand Cardiovasc J. 2016;50(5-6):311–316.
- van Ryn J, Hauel N, Waldman L, Wienen W. Dabigatran inhibits both clot-bound and fluid-phase thrombin in vitro: comparison to heparin and hirudin [abstract 570]. Arterioscler Thromb Vasc Biol. 2008;28:e136-e137.
- Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Vinholt PJ, Nielsen C, Söderström AC, Brandes A, Nybo M. Dabigatran reduces thrombin-induced platelet aggregation and activation in a dose-dependent manner. J Thromb Thrombolysis. 2017;44(2):216–222.
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 medi- ate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887.
- Harrison P, Mackie I, Mumford A, Briggs C, Liesner R, Winter M, Machin S, British Committee for Standards in Haematology. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155(1):30–44.
- Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30(12):2341–2349.
- Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206–232.
- Sreiff MB, Agnelli G, Connors JM, Crowther M, Eichinger S, Lopes R, McBane RD, Moll S, Ansell J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis. 2016;41(1):32–67.
- Olivier CB, Weik P, Meyer M, Weber S, Anto-Michel N, Diehl P, Zhou Q, Geisen U, Bode C, Moser M. TRAP-induced platelet aggregation is enhanced in cardiovascular patients receiving dabigatran. Thromb Res. 2016;138:63–68.
- Perzborn E, Heitmeier S, Buetehorn U, Laux V. Direct thrombin inhibitors, but not the direct factor Xa inhibitor rivaroxaban, increase tissue factor-induced hypercoagulability in vitro and in vivo. J Thromb Haemost. 2014;12(7):1054–1065.
- Martischnig AM, Mehilli J, Pollak J, Petzold T, Fiedler AK, Mayer K, Schulz-Schüpke S, Sibbing D, Massberg S, Kastrati A, Sarafoff N. Impact of Dabigatran versus Phenprocoumon on ADP Induced Platelet Aggregation in Patients with Atrial Fibrillation with or without Concomitant Clopidogrel Therapy (the Dabi-ADP-1 and Dabi-ADP-2 Trials). Biomed Res Int. 2015;2015:798486.
- Nehaj F, Sokol J, Ivankova J, Mokan M, Kovar F, Stasko J, Mokan M. First Evidence: TRAP-Induced Platelet Aggregation Is Reduced in Patients Receiving Xabans. Clin Appl Thromb Hemost. 2017;1:1076029617734310. [Epub ahead of print].
- Nehaj F, Sokol J, Mokan M, Ivankova J, Mokan M. Thrombin Receptor Agonist Peptide-Induced Platelet Aggregation Is Reduced in Patients Receiving Dabigatran. Clin Appl Thromb Hemost. 2018;24(2):268–272.