 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives. Guidelines of the European Society of Cardiology (ESC) recommend that ferritin and transferrin saturation should be tested in chronic heart failure (HF) and state that iron treatment with ferric carboxymaltose should be considered in HF patients with iron deficiency to alleviate symptoms and improve exercise tolerance and quality of life. This study evaluates the cost effectiveness of the implementation of this recommendation in four Nordic countries (Denmark, Finland, Norway, and Sweden). Design. We performed a cost-utility analysis comparing ferric carboxymaltose treatment with placebo over a one-year time period in each country. Data on healthcare resource use and health outcomes were taken from the CONFIRM-HF study and combined with country-specific unit costs. Differences in per-patient costs and quality-adjusted life years (QALYs) were calculated. Results. QALYs were higher (increase of 0.050 QALYs per patient) in the iron-treated group compared with placebo. Per-patient costs were lower in all countries (with reductions ranging from €36 to €484). Fewer hospitalizations were one key driver of these results. Another important driver was how well the new routines for iron treatment can be integrated into the current healthcare management of HF. A sensitivity analysis confirmed the results to be robust. Conclusions. Iron deficiency therapy in HF with ferric carboxymaltose compared with placebo is estimated to both improve health-related quality of life and save healthcare costs in all Nordic countries. A well-organized healthcare management of HF patients can enable the implementation of ESC-recommended treatment of iron deficiency without need for additional resources.
Introduction
Chronic heart failure (HF) affects around 2% of the population, but it is mainly present in older people affecting 6–10% of the population aged 65 years and older [Citation1, Citation2]. The condition impairs patients’ quality of life, physical capacity, as well as cognitive health [Citation3, Citation4], and is linked to high mortality and frequent hospitalizations [Citation5]. Its treatment requires a large share of healthcare resources [Citation6].
Until recently, iron deficiency was an overlooked and hence untreated comorbidity in HF, even though it seems to affect up to half of all HF patients with reduced ejection fraction. The prevalence increases with disease severity of HF [Citation7, Citation8]. Mounting evidence and understanding of the role of iron deficiency in HF has led to the acknowledgment of iron deficiency as a comorbidity in HF by the European Society of Cardiology (ESC) in the guidelines on heart failure published in 2012 [Citation9]. In the most recent ESC Heart Failure Association guidelines from 2016, the ESC recommends regular measurement of ferritin and transferrin saturation on top of hemoglobin [Citation10]. In addition, iron therapy with the intravenous iron preparation ferric carboxymaltose (FCM) should be considered in HF patients with iron deficiency with a IIa class of recommendation, level of evidence A.
FCM has been approved and used for the treatment of iron deficiency in non-HF patients for over a decade. With regard to HF, the FAIR-HF study was the first large-scale phase III trial to evaluate the treatment of iron deficiency (defined as ferritin level <100 µg/L or 100–299 µg/L if transferrin saturation <20%) with FCM in HF patients [Citation11]. In this 24-week parallel comparison between FCM and placebo, FCM was linked to significant improvements in New York Heart Association (NYHA) functional class, self-reported Patient Global Assessment (PGA), Six-Minute Walk Test (6MWT), and health-related quality of life (HRQoL) in both anemic and non-anemic patients. Moreover, health economic analyses modeled on this study indicated a favorable cost-effectiveness profile in, e.g., Spain, Sweden, and the UK [Citation12–14].
The CONFIRM-HF study was a double-blind, placebo-controlled, randomized phase III trial in which 304 chronic HF patients with NYHA class II or III with iron deficiency were randomly assigned to receive either FCM or saline in a 1:1 ratio [Citation15]. Over the 52-week follow-up period, significant improvements in 6MWT, the primary endpoint, as well as in the secondary endpoints of NYHA class, PGA, HRQoL, Fatigue Score on the Kansas City Cardiomyopathy Questionnaire, and rate of HF-related and cardiovascular-related hospitalizations compared with placebo were reported.
The administration and dosage of FCM in the CONFIRM-HF study differed from the FAIR-HF study and is the basis for the currently approved dosing scheme. Moreover, the follow-up time in CONFIRM-HF was much longer. Therefore, we chose to utilize these data in our health economic analysis. The aim of this analysis was to assess the cost effectiveness of implementing the ESC recommendation to treat iron deficiency in HF with FCM in the four major Nordic countries; Denmark, Finland, Norway, and Sweden.
Methods
A cost-utility analysis was performed to evaluate the cost effectiveness of FCM treatment in iron-deficient HF patients. The cost-utility analysis is a health economic evaluation method used when health outcomes are measured with a unified generic utility measure, such as quality-adjusted life years (QALYs), in both the treatment group and the comparison group and the associated costs estimated in these groups [Citation16]. In current clinical practice, iron deficiency in HF remains most often undetected and thus untreated in the Nordic countries. The appropriate comparator for FCM treatment of iron deficiency in HF in this analysis is, therefore, no treatment.
In this analysis, health outcomes were measured as HRQoL and expressed in QALYs. Separate cost analyses were carried out for Denmark, Finland, Norway, and Sweden to account for country-specific conditions. National unit costs for healthcare resources, based on the Nordic Diagnosis Related Groups (DRG) system, are shown in [Citation17–22]. The average tendered price per country for FCM in 2017 was provided by Vifor Pharma Nordiska, Stockholm, Sweden. Costs are presented in 2017 euros (€), adjusted to 2017 price levels using the consumer price index when necessary [Citation23]. The average exchange rates in 2017 were €1 = DKK 7.4386, €1 = NOK 9.3270, and €1 = SEK 9.6351 [Citation24]. The time horizon was 52 weeks in the analysis, corresponding to the follow-up period in the CONFIRM-HF study. Given this time horizon, health outcomes and costs were not discounted. The result of the cost-utility analysis is an incremental cost-effectiveness ratio (ICER), , which interrelates the difference in costs of treatment with FCM and placebo in each country with the difference in HRQoL.
Table 1. Unit costs of resources (2017 price levels in euros and local currencies), [Citation18, Citation19, Citation21–23].
The cost-utility analysis was modeled on the setup of the CONFIRM-HF study but incorporated certain adjustments to take into account the ESC guidelines and to better reflect the envisaged clinical practice in the Nordic countries.
Health outcomes
In the CONFIRM-HF study, HRQoL was measured with the EuroQoL Visual Analogue Scale (EQ-VAS) at baseline and at weeks 6, 12, 24, 36, and 52. Based on published aggregate figures, we calculated utility values, i.e., an index between 0 and 1, where 0 represents death and 1 best possible health. QALYs for each study group were obtained by multiplying utility values with the appropriate time interval in between the assessment time points, assuming that changes in utility occur in the middle of these intervals. However, the preferred measure for HRQoL in cost-utility analysis is based on the application of a validated value set to the EuroQoL five dimensions (EQ-5D) questionnaire to calculate QALYs. As EQ-5D was not measured in the CONFIRM-HF study, we drew on published data from the FAIR-HF study to provide an estimate in the following manner. QALYs based on both EQ-VAS and EQ-5D applying the UK time trade-off value set [Citation25], which is also commonly applied in Nordic cost-effectiveness analyses, have been previously published for the FAIR-HF study [Citation13]. We used the relationship between these two values and combined it with the calculated EQ-VAS-based value for QALYs from the CONFIRM-HF study to estimate QALYs based on EQ-5D.
Resource use
We built a model to measure the use of healthcare resources based on the setup of the CONFIRM-HF study. To derive a realistic base case scenario that reflects the envisaged clinical practice in the Nordic countries, we also drew on the ESC guidelines on HF management. Four resource categories which are affected by FCM treatment were included in the analysis; diagnostic tests, FCM, administration of FCM, hospitalization. details all resource parameters used in the analysis.
Table 2. Parameter values for the base case scenario and the sensitivity analysis.
In clinical practice, FCM must only be administered if the diagnosis of iron deficiency is based on laboratory tests (we include ferritin, transferrin saturation, and hemoglobin). According to the ESC guidelines, HF patients should be routinely screened for the presence of iron deficiency. Also, iron deficiency is a non-acute condition. The assessment of the iron deficiency status can thus be planned and take place in conjunction with a regular healthcare visit for monitoring HF patients. In the base case scenario, we only included the costs for laboratory tests prior to and after the initial treatment with FCM in the FCM group. If an additional treatment with FCM is required, costs for follow-up laboratory tests were also included. In the FCM group, we included the costs for diagnosing all HF patients intended for FCM treatment (and not only those with a positive diagnosis), assuming a prevalence of iron deficiency of 50% in HF [Citation8]. Since healthcare visits for performing these diagnostic tests can be planned, we did not include the costs for a separate visit in the base case.
Once the iron deficiency is diagnosed in HF patients, the ESC guidelines recommend treatment with FCM. Correct dosing of FCM is determined by a patient’s body weight and hemoglobin level resulting in six possible dosing combinations (assuming no patients with less than 35 kg body weight). This dosing scheme was also used in the CONFIRM-HF study for the initial treatment with FCM. In the base case scenario, we relied on the initial allocation of patients in the FCM group in the CONFIRM-HF study across the six dosing combinations; see . If administered as an intravenous infusion, the maximum single dose of FCM is 1000 mg of iron per day. If the cumulative dose to correct iron deficiency exceeds 1000 mg of iron, at least one week of time must pass between the first and the second infusion. In the CONFIRM-HF study, those patients that needed a second infusion as part of their initial treatment received it six weeks after the first infusion. The administration of an infusion of up to 1000 mg takes 15 min, and patients have to remain under observation for 30 min after an infusion. In the base case scenario, we assumed that the first infusion can be planned and hence take place in conjunction with a regular healthcare visit at a cardiology department, requiring no extra costs for this visit. However, we did include the costs for a healthcare visit to receive the second infusion for those patients requiring one, as it might be more difficult to combine it with an unrelated visit in clinical practice.
The CONFIRM-HF study showed that the correction of the iron deficiency status for most patients was achieved after the initial treatment and maintained until week 52. At the first follow-up visit after the initial treatment at week 12, 18.9% of the patients still had iron deficiency. In clinical practice, those patients would once again receive FCM according to the dosing scheme. In the base case scenario, we assumed that half of those patients require a cumulative iron dose of 1500 mg, and a quarter of patients 500 mg and 1000 mg, respectively. This differs from the CONFIRM-HF study in which patients uniformly received 500 mg as long as iron deficiency was present at weeks 12, 24, and 36. In line with the approach for the initial treatment, we assumed that the first infusion of the additional treatment can be planned and requires no separate healthcare visit, whereas we did include the costs for a visit to receive the second infusion.
In the CONFIRM-HF study, the numbers of both cardiovascular-related and HF-related hospitalizations were significantly (p-values of 0.0174 and 0.0021, respectively) lower in the treatment group compared with the placebo group. In the FCM group, 26 cardiovascular-related hospitalizations were recorded during the whole study period (corresponding to a mean annual rate of 0.17 hospitalizations per patient), and 51 hospitalizations in the placebo group (0.34 hospitalizations per patient). In the base case scenario, we used these hospitalization rates.
Sensitivity analysis
A deterministic sensitivity analysis was conducted to examine the robustness of the resource use parameters and the underlying assumptions in the base case. One of the critical assumptions is whether separate healthcare visits are required for performing diagnostic tests and for all administrations of FCM. In clinical practice, this will depend on how well the new routines for the screening of iron deficiency status and iron treatment can be integrated into the current healthcare management of HF patients. We simulated various scenarios to quantify the impact of the flexibility of healthcare management. Furthermore, we tested the impact of the dosing of FCM based on the initial patient allocation, the share of patients requiring an additional FCM treatment, the price of FCM, different definitions of the hospitalization rate, as well as the computation of and the difference in QALYs. We also re-ran the whole analysis based on the median patient in the CONFIRM-HF study.
Results
In general, costs of care were different between the Nordic countries in 2017 () with the most expensive care given in Norway for an HF-related hospital admission and the least expensive one in Finland. A similar cost pattern was seen for outpatient visits at a cardiology department.
Results of the cost-utility analysis are shown in . As regards costs, treatment with FCM resulted in a decrease in total costs per patient of €298 in Denmark, €36 in Finland, €484 in Norway, and €379 in Sweden compared with no treatment of iron deficiency in HF patients over the 52-week study period in the base case scenario. illustrates the total costs per patient broken down by the four cost categories; see also in the online Appendix (Supplemental Material). The estimated reduction in costs for hospitalization in the FCM group was in all countries large enough to offset the increase in costs due to FCM (which amounts to between €204 and €237 in all countries), FCM’s administration, as well as diagnostic tests.
Figure 1. Mean costs per patient (in 2017 €) in the treatment group (FCM) and the placebo group (no treatment) in the base case scenario.
FCM: ferric carboxymaltose; €: euros.
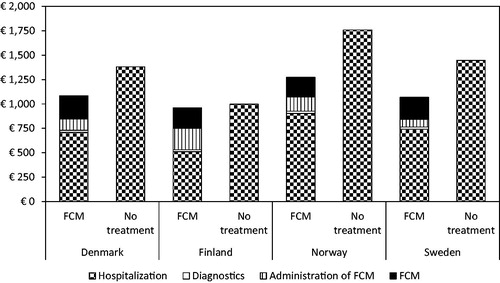
Table 3. Results of the cost-utility analysis and the sensitivity analysis (in 2017 €).
In terms of HRQoL, FCM treatment resulted in a QALY gain of 0.050 per patient (95% confidence interval (CI) 0.003–0.098) compared with no treatment during the study period, according to the extrapolation for EQ-5D; see . Based on EQ-VAS, the QALY gain amounted to 0.031 (95% CI 0.002–0.061).
As a result, FCM treatment of iron deficiency in HF patients was both cost saving and more effective in all four Nordic countries. This means that FCM treatment dominated no treatment in the base case scenario, and hence no ICER expressed in costs per QALY could be calculated for this point estimate in the cost-effectiveness plane.
The results of the sensitivity analysis confirmed the base case results to be robust and are presented in detail in and in in the online Appendix (Supplemental Material). The scenarios considered in illustrate the importance of a well-organized HF management, which has enough capacity and flexibility to implement the ESC guidelines in a cost-saving manner. For instance, if all healthcare visits required for FCM’s administration are assumed to occur unplanned and hence cause additional costs, the costs savings are roughly cut in half in Denmark, Norway, and Sweden, while in Finland FCM treatment becomes more expensive than no treatment. A similar pattern can be observed when all healthcare visits related to performing diagnostic tests are assumed to occur unplanned instead of in conjunction with regular healthcare visits. If all types of healthcare visits occur unplanned, FCM treatment still reduces costs in Norway and Sweden compared to no treatment, while in Denmark costs are equally high, and in Finland costs increase.
Furthermore, the hospitalization rate was a key driver of the result. If no difference in the hospitalization rate between the study groups was assumed (see ), the total costs were naturally higher in the FCM group than in the placebo group. Yet even in this scenario FCM treatment still had a favorable cost-effectiveness profile, as the ICER was around €6500–€9,000 per QALY in all countries, which is far below the typical cost-effectiveness thresholds (around €45,000 per QALY) in the Nordic countries.
Discussion
In this analysis, we demonstrated that FCM is cost-effective across the four major Nordic countries. Improving the quality of life is a very important aspect of HF management as is reducing HF-related hospitalizations, which are linked to worse prognosis. It has been firmly established that modern HF medication, as well as device therapy, reduce morbidity and mortality [Citation10], but also that such therapies are not optimally implemented in real life, both according to international surveys [Citation26] and registries [Citation27]. Iron deficiency has only recently been recognized as a comorbidity in HF which should be investigated and treated. Therefore, the National Norwegian Heart Failure Registry (NNHFR) and the Swedish Heart Failure Registry (SwedeHF) have added ferritin, transferrin saturation, and FCM treatment amongst entered parameters which can in the future be used to follow up the implementation of ESC guidelines regarding diagnosis and treatment of iron deficiency in HF patients.
The 2016 ESC guidelines specifically recommend treatment of iron deficiency with FCM based on the two large-scale randomized controlled clinical trials [Citation11, Citation15]. No comparable trials have yet been completed for other parenteral iron formulations in iron-deficient HF [Citation28]. Oral iron therapy is usually the first-line treatment for iron deficiency in therapeutic areas other than HF because of convenience and low cost. The effectiveness of oral iron therapy in iron-deficient HF has been recently evaluated in a randomized clinical trial involving 225 patients [Citation29], but found no differences in exercise capacity or other health-related outcomes between the group receiving oral iron polysaccharide compared with placebo over a 16-week study period.
The generalizability of the results of the cost-utility analysis should be judged against the backdrop of the representativeness of the study population in the CONFIRM-HF study. The intention-to-treat population with post-baseline records in the CONFIRM-HF study was composed of 301 patients recruited across nine European countries (including some patients in Sweden but not in other Nordic countries) [Citation15]. compares baseline patient characteristics from the CONFIRM-HF study with patients managed in outpatient hospital care from the Danish Heart Failure Registry (DHFR), the Norwegian NNHFR, and the Swedish SwedeHF [Citation30–32]. Even though very limited comparisons can be made between large registries and an RCT, the patients are fairly similar in many respects although the share of female patients and the prevalence of concomitant diseases are lower in all registries as well as Swedish patients seem to be older.
Table 4. Characteristics of the CONFIRM-HF study population at baseline and of patients in national HF registries.
The economic burden of chronic HF is high and has been estimated to equal about 2% of the total healthcare budget in Western countries [Citation1, Citation33]. Following the 2016 ESC guidelines, we showed that the recommendation of treating iron deficiency in HF with FCM can be implemented in a cost-saving manner. This result rested partly on the fact that iron deficiency is a non-acute condition. Therefore, its diagnosis, treatment, and follow-up can in principal be planned and take place in conjunction with regular healthcare visits, as HF patients typically make several healthcare visits per year to monitor and adjust treatment and comorbidities [Citation33]. However, the scenarios considered in the sensitivity analysis showed that even if some additional healthcare visits were required, FCM treatment would still be cost saving in most countries. The economic burden of HF thus need not rise due to the introduction of a new additional treatment such as FCM. Importantly, HRQoL, symptoms, and exercise tolerance of iron-deficient HF patients can improve.
Hospitalizations were another a key driver of the result. This is not surprising, since HF is the most common cause of hospitalization in patients aged over 65 years [Citation32], and hospitalization is by far the greatest cost component of total healthcare expenditure for HF [Citation6, Citation33]. Even though the CONFIRM-HF study was not powered to detect statistically significant differences in hospitalizations, there was a significant and quantitatively large reduction in cardiovascular-related, HF-related, and all-cause hospitalizations during the study period. A pooled analysis of four clinical trials of FCM treatment in iron-deficient HF involving 839 patients also demonstrated a similar and significant reduction in the number of hospitalizations [Citation34]. Randomized controlled studies dimensioned to evaluate hospitalization rates and also mortality will allow for improved evaluations of cost per QALY saved. Currently, three such studies, HEART-FID (clinicaltrials.gov NCT03037931), FAIR-HF2 (NCT03036462), and Affirm-AHF (NCT02937454), are ongoing.
Limitations
Some limitations in our analysis originated from the design of the CONFIRM-HF study. The analysis was restricted to a healthcare perspective in the Nordic countries. In health economic analyses, it is desirable to apply a wider societal perspective. Indirect costs outside the remit of the healthcare payer, such as morbidity-related productivity loss and informal care, could not be included due to lack of data. However, given the advanced age of the typical HF patient, changes in morbidity-related productivity loss would be small, as most patients are already retired. The need for informal care might be reduced by FCM treatment if patient health and physical capacity improve as observed in the clinical trials. We did not include healthcare resource use in connection with the treatment of adverse events and death in the analysis, as their occurrence was similar in each study group in the CONFIRM-HF study.
Another limitation of this study is the lack of directly available EQ-5D data. These data would be the preferred generic measure to calculate changes in HRQoL in terms of QALYs. In this study, it was only possible to infer changes in EQ-5D-based QALYs indirectly via changes in EQ-VAS.
The way FCM was administered after the initial treatment constitutes another limitation. As mentioned above, at the first follow-up visit at week 12 the 18.9% of patients who were still iron-deficient received a maintenance dose of 500 mg of FCM instead of the appropriate dose according to the approved dosing scheme. This was also done at week 24 and week 36. However, more than 75% of patients required at most two injections of FCM during the whole study period. In fact, the median patient only required two injections of in total 1,500 mg of FCM in connection with the initial treatment to remedy iron deficiency and maintain this status throughout the study period. This corroborates a long-lasting effect of FCM for the great majority of patients based on an administration mode relevant for clinical practice. Nonetheless, evidence of the effects of FCM on health outcomes and healthcare resource use beyond the first year of treatment would be desirable.
Conclusion
The treatment of iron deficiency in chronic HF with FCM compared with placebo was estimated to be both HRQoL-improving and cost-saving in all four Nordic countries. A reduction in hospitalizations was one key driver of these results. Another key driver was how well the new routines for measurement of iron deficiency status and iron treatment can be integrated into the current healthcare management of HF patients. If these routines can at least be partly combined with regular healthcare visits of HF patients, the ESC-recommended treatment of iron deficiency can be implemented without need for additional healthcare resources.
Supplemental Material
Download MS Word (54.3 KB)Disclosure statement
FCM is marketed as Ferinject in the Nordic countries by Vifor Pharma Nordiska AB, Stockholm, Sweden. TH is an employee of IHE. DCA is an employee of Vifor Pharma Nordiska AB. CL receives no funding from Vifor Pharma Nordiska AB. The authors alone were responsible for the content and writing of the article. The authors declare no competing interests.
Additional information
Funding
References
- McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889.
- Zarrinkoub R, Wettermark B, Wandell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002.
- Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23:464–475.
- Hobbs FD, Kenkre JE, Roalfe AK, et al. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–1876.
- Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133.
- Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace. 2011;13:ii13–ii17.
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880.
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582 e3.
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847.
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448.
- Comin-Colet J, Rubio-Rodriguez D, Rubio-Terres C, et al. A Cost-Effectiveness Analysis of Ferric Carboxymaltose in Patients with Iron Deficiency and Chronic Heart Failure in Spain. Rev Esp Cardiol (Engl Ed). 2015;68:846–851.
- Gutzwiller FS, Schwenkglenks M, Blank PR, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR-HF trial: an analysis for the UK. Eur J Heart Fail. 2012;14:782–790.
- Hofmarcher T, Borg S. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ. 2015;18:492–501.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668.
- Drummond M. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
- Purchasing power parities (PPPs), price level indices and real expenditures for ESA 2010 aggregates [prc_ppp_ind] [Internet]. Luxembourg: Eurostat; [February 2, 2018]. Available from: http://ec.europa.eu/eurostat/data/database
- Helsedirektoratet. Innsatsstyrt Finansiering 2017 [Investment-based funding 2017]. Oslo: Helsedirektoratet, 2016.
- Kapiainen S, Väisänen A, Haula T. Terveyden- ja Sosiaalihuollon Yksikkökustannukset Suomessa Vuonna 2011 [Unit Costs of Health and Social Care in Finland in 2011]. Tampere: Terveyden ja hyvinvoinnin laitos, 2014.
- Region Skåne Medicinsk service - Labmedicin - Pris 2017 - Klinisk Kemi [Medical Service - Laboratory Medicine - Price 2017 - Clinical Chemistry]. Lund/Malmö: Region Skåne, 2016.
- Sundhedsdatastyrelsen. Takstsystem 2017 - Vejledning [Tariff System 2017 - Guidance]. Copenhagen: Sundhedsdatastyrelsen, 2016.
- Södra Regionvårdsnämnden Regionala Priser Och Ersättningar För Södra Sjukvårdsregionen 2017 [Regional Prices and Compensation for the Southern Health Care Region 2017]. Lund/Malmö: Södra Regionvårdsnämnden, 2016.
- HICP (2015 = 100) - Annual Data (average Index and Rate of Change) [Prc_hicp_aind] [Internet]. Luxembourg: Eurostat; [February 2, 2018]. Available from: http://ec.europa.eu/eurostat/data/database
- Euro foreign exchange reference rates [Internet]. Frankfurt: European Central Bank; [March 8, 2018]. Available from: http://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/index.en.html
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108.
- Maggioni AP, Van Gool K, Biondi N, et al. Appropriateness of prescriptions of recommended treatments in organisation for economic co-operation and development health systems: findings based on the long-term registry of the European Society of Cardiology on Heart Failure. Value Health. 2015;18:1098–1104.
- Thorvaldsen T, Benson L, Dahlstrom U, et al. Use of evidence-based therapy and survival in heart failure in Sweden 2003-2012. Eur J Heart Fail. 2016;18:503–511.
- Drozd M, Jankowska EA, Banasiak W, et al. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs. 2017;17:183–201.
- Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA. 2017;317:1958–1966.
- Kompetencecenter for Klinisk Kvalitet og Sundhedsinformatik Vest. Dansk Hjertesvigtdatabase - Årsrapport 2014 - Version 1.1 [Danish Heart Failure Database - Annual Report 2014 - Version 1.1]. Aarhus: Kompetencecenter for Klinisk Kvalitet og Sundhedsinformatik Vest, 2015.
- Nasjonalt sekretariat for Norsk hjertesviktregister. Norsk hjertesviktregister - Årsrapport 2016 [Norwegian Heart Failure Register - Annual Report 2016]. Trondheim: Nasjonalt sekretariat for Norsk hjertesviktregister, 2017.
- Vasko P, Jonsson Å, Dahlström U, et al. Årsrapport RiksSvikt - 2015 års resultat [Annual Report SwedeHF - 2015 results]. 2017.
- Agvall B, Borgquist L, Foldevi M, et al. Cost of heart failure in Swedish primary healthcare. Scand J Prim Health Care. 2005;23:227–232.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–133.
