Abstract
Background. In families with an inherited form of non-syndromic thoracic aortic disease (TAAD), aortic diameter alone is not a reliable marker for disease occurrence or progression. To identify other parameters of aortic function, we studied aortic stiffness in families with TAAD. We also compared diameter measurements obtained by transthoracic echocardiography (TTE) and magnetic resonance imaging (MRI). Methods. Seven families, including 116 individuals, with non-syndromic TAAD, were studied. The aortic diameter was measured by TTE and MRI. Aortic stiffness was assessed as local distensibility in the ascending aorta and as regional and global pulse wave velocity (PWV). Individuals with a dilated thoracic aorta (n = 21) were compared with those without aortic dilatation (n = 95). Results. Ascending aortic diameter measured by TTE strongly correlated with the diameter measured by MRI (r2 = 0.93). The individuals with dilated aortas were older than those without dilatation (49 vs 37 years old). Ascending aortic diameter increased and distensibility decreased with increasing age; while, PWV increased with age and diameter. Some young subjects without aortic dilatation showed increased aortic stiffness. Individuals with a dilated thoracic aorta had significantly higher PWV and lower distensibility, measured by MRI than individuals without dilatation. Conclusions. Diameters measured with TTE agree with those measured by MRI. Aortic stiffness might be a complementary marker for aortic disease and progression when used with aortic diameter, especially in young individuals.
Introduction
Current guidelines for thoracic aortic disease recommend screening first-degree relatives of patients with thoracic aortic aneurysms and dissections (TAAD) [Citation1,Citation2]. In familial thoracic aortic aneurysms and dissections (FTAAD), screening of both first- and second-degree relatives is recommended [Citation1]. Although a large number of subjects must be screened, a substantial number of individuals with a dilated thoracic aorta can be found by following these guidelines [Citation3]. The aortic diameter is often used for the diagnosis and risk stratification in FTAAD, but because dissections may occur at smaller diameters than in sporadic cases, it is not always useful and other diagnostic tools are needed.
The disease-causing genetic variant can only be identified in about 20% of inherited cases [Citation4]. Therefore, in the majority of patients, the diagnosis and treatment strategy rely on measuring the thoracic aortic diameter. Many individuals, especially at young ages, may have a normal aortic diameter even if they are carriers of the disease-causing gene; therefore, they are likely to be missed during screening [Citation3]. It is currently impossible to differentiate carriers from non-carriers, i.e. persons at risk for dissection when the thoracic aorta has a normal diameter.
Studies have suggested that subjects with inherited forms of aortic aneurysms are younger and have smaller diameters when dissections occur than subjects with sporadic forms [Citation4]. Carriers of certain gene mutations may dissect at normal or near normal aortic dimensions [Citation3,Citation4]. It is unclear when mutation-carriers and individuals with a dilated thoracic aorta in these families should undergo elective prophylactic interventions. Other parameters beyond diameter, e.g. aortic stiffness, might be helpful in the surveillance of individuals with dilated thoracic aortas and when determining the timing of elective interventions.
There is an ongoing discussion about which imaging modality should be used for screening and if the measurements from different modalities are comparable [Citation5]. Computed tomography is widely available and fast to perform, but exposes the patient to ionizing radiation and intravenous contrast agent. Magnetic resonance imaging (MRI) does not expose the patient to radiation but takes longer to perform and it is not as available as computed tomography or transthoracic echocardiography (TTE). Furthermore, approximately 5% of patients cannot undergo MRI due to claustrophobia. The TTE is widely available, easy to perform, and does not expose the patient to radiation or contrast agents. The descending thoracic aorta is difficult to investigate with ultrasound, but in the vast majority of the patients the disease is localized to the ascending aorta (AoA).
In an attempt to identify clinically useful parameters for diagnosing thoracic aortic disease, we have studied aortic elastic properties using three different modalities (TTE, MRI, and Arteriograph®) in families with TAAD. In addition, we compared the AoA diameters measured by TTE and MRI. The research questions addressed were (1) Are the diameter measurements obtained by TTE and MRI comparable in clinical practice? (2) Do measurements of aortic stiffness provide additional information in the surveillance of individuals with suspected thoracic aortic disease?
Material and methods
Study population
In these seven families, consisting of 135 individuals, eleven individuals had died due to aortic dissection or rupture and eleven persons had a previous aortic dissection (six individuals) or elective prophylactic operation (five individuals). An additional 21 individuals had a dilatation of the sinuses of Valsalva or AoA. No dilatation of the descending aorta was identified. The demographic data of the individuals are shown in . In four individuals, it was not possible to measure the aortic diameter or distensibility by TTE because the entire AoA could not be visualized or the image quality was poor. MRI was not performed in six individuals; four due to claustrophobia, one due to an implantable cardiac defibrillator and one due to pregnancy. In two individuals, the MRI image quality did not allow measuring the AoA diameter and in two other individuals, the PWV could not be measured with MRI.
The study population consisted of seven consecutive families with FTAAD referred to the Centre for Cardiovascular Genetics, Umeå University Hospital. The genetic tests for FTAAD in probands were negative. Each family had a history of at least two individuals with verified TAAD, and eleven individuals from these families had died from aortic dissection. All first- and second-degree family members older than 18 years and without TAAD were offered participation in the study. The families consisted of 135 members, of whom eleven living individuals had an aortic dissection or were prophylactically operated on. Eight individuals did not participate in the study. Six of them, mainly adolescents, declined participation; one individual living in another city did not have the opportunity to participate; and one individual was not offered participation because of a serious concomitant disease. The remaining 116 family members formed the initial study population and were screened for thoracic aortic aneurysms. Among these individuals, nineteen had a dilated aorta and two had a previously known dilatation of the AoA, as reported in an earlier study [Citation3]. The population in the present study consisted of the 21 individuals with dilated aortas (two previously known and 19 new cases) and 95 relatives with normal aortic dimensions. No dilatation of the descending aorta was identified. The demographic data of the individuals are shown in . In four individuals, it was not possible to measure the aortic diameter or distensibility by TTE because the entire AoA could not be visualized or the image quality was poor. MRI was not performed in six individuals; four due to claustrophobia, one due to an implantable cardiac defibrillator and one due to pregnancy. In two individuals, the MRI image quality did not allow measuring the AoA diameter and in two other individuals, the PWV could not be measured with MRI. The subjects were judged to have arterial hypertension or coronary artery disease if they had medical therapy for these diagnoses. The regional ethical review board at Umeå University approved the study. Signed informed consent was obtained from the participants after both oral and written information was provided.
Table 1. Demographic data, distensibility, and PWV of the study population.
Measurements
TTE
The TTE was performed using a Vivid 7 (GE Medical Systems, Horten, Norway) echocardiography machine. Aortic diameter was measured from the parasternal long-axis view at the sinuses of Valsalva and at the widest level of the AoA. All measurements were made in end-diastole and considered the inner edge-to-inner edge distance from the parasternal long-axis view. Measurements were made in M-mode after verifying correct positioning and alignment in the two-dimensional image. For analysis of aortic distensibility, the maximal systolic diameter was measured in M-mode at the same level in the AoA. The average of three measurements in different cardiac cycles was calculated. Diameters were indexed according to age, sex, and body surface area. Aortic distensibility was calculated as (AoAmax − AoAmin)/(AoAmin × PP), where AoAmax is the ascending aortic diameter in systole, AoAmin is the ascending aortic diameter in diastole, and PP is the pulse pressure (systole minus diastole) calculated from the blood pressure measured in the left arm at the end of the echocardiographic examination. All examinations were performed by one of two sonographers, then reviewed and analyzed offline by one experienced investigator. Normal values for aortic diameters, adjusted for height, weight, gender, and age, published by Mirea et al. were used as reference echocardiographic diameters [Citation6]. Ascending aortic diameters exceeding two standard deviations were defined as dilated.
MRI
MRI was performed with the Achieva 3.0T MRI system (Philips, Best, The Netherlands) with the patient in the supine position. All imaging was electrocardiography gated with a three-lead vector electrocardiogram and acquired during an expiratory breath hold. Localizer sequences were followed by transaxial T1- and T2-weighted “black blood” sequences over the heart and the great vessels. Phase velocity maps were obtained in the same imaging planes. The internal diameters of the ascending and descending aorta were measured at the level of the pulmonary bifurcation in two perpendicular measurements, using the smallest diameter. The measurements were made by a single reader without knowledge of the diameters obtained via echocardiography. Data from Davis et al. [Citation7] were used as reference values for MRI measurements of the ascending and descending aorta.
Cross-sectional lumen areas of the ascending and descending aorta were determined throughout the cardiac cycle using a semi-automated contouring method (Segment® v2.0 R4377, MedViso, Lund, Sweden) at the level of pulmonary bifurcation and edited when needed [Citation8]. All tracings were reviewed and edited by one observer. Systolic and diastolic arterial blood pressures were measured in the left arm at the beginning of the flow sequences. Aortic distensibility was calculated with the formula D = (AoAmax − AoAmin)/(AoAmin × PP), where AoAmax is the largest area in ascending aorta (systole), AoAmin is the smallest area (diastole) and PP is the pulse pressure. The pulse wave velocity (PWV) analysis tool of the Segment program was used to calculate the PWV from the MRI images.
PWV
Arterial stiffness was measured with Arteriograph® (TensioMed, Budapest, Hungary) after the subject rested for 5 min in the supine position. Arteriograph® enables pulse wave analysis using an oscillometric method [Citation9]. An inflated cuff on the upper right arm registers pulsatile pressure changes in the brachial artery beneath the cuff. The pressure changes are passed on to a computer and recorded and analyzed as pulse waves. The difference in time between the beginning of the first wave and the beginning of the second reflected wave is related to the distance from the jugulum to the symphysis, resulting in the PWV [Citation9]. The method has been validated against invasive and other non-invasive methods to measure PWV [Citation10–12]. The PWV was used as a marker of aortic stiffness.
Statistical analysis
All data were analyzed using SPSS software version 22 (IBM, Armonk, NY, USA). Pearson´s correlation and Bland-Altman plots were used to analyze the agreement between aortic diameters measured by TTE and MRI. An independent-sample Mann–Whitney U-test was used to compare distensibility and PWV between the groups with and without dilatation. To adjust for the finding that individuals with dilated aortas were older than those without dilatation, we then analyzed distensibility and PWV in separate multiple linear regression models, including age and diameter as continuous covariates and dilated aorta as a binary variable. To analyze if the relationship between the presence of dilatation and distensibility/PWV varies with age or diameter, two-way interactions (dilatation*age, dilatation*diameter) were included. p values less than 0.05 were considered statistically significant.
Results
Aortic diameters measured by TTE and MRI
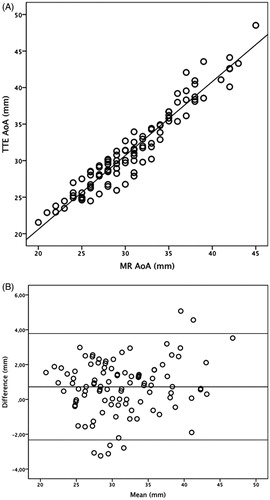
The diameters measured by TTE and MRI strongly correlated (r2 = 0.93, ). The Bland-Altman analysis showed that the mean difference in diameters measured by TTE was 0.72 mm (95% CI 0.41–1.02 mm, SD 1.56) larger than those measured with MRI (). The difference in diameter between the methods can probably be explained by the different way the measurements were made. With TTE, the largest diameter in the AoA was measured. With MRI, the diameter was measured at a fixed position at the level of the bifurcation of the pulmonary artery. Two cross-sectional diameters were measured and the smaller diameter was reported.
Figure 1. Linear regression, r2 = 0.93 (a) and Bland–Altman plot (b) for diameters of the ascending aorta measured by TTE and MRI. The individual mean aortic diameters measured with TTE and MRI are shown on the x-axis and the deviation from the mean the y-axis. The middle horizontal line shows the mean difference between TTE and MRI (0.72 mm), i.e. TTE systematically reports slightly higher values than MRI.
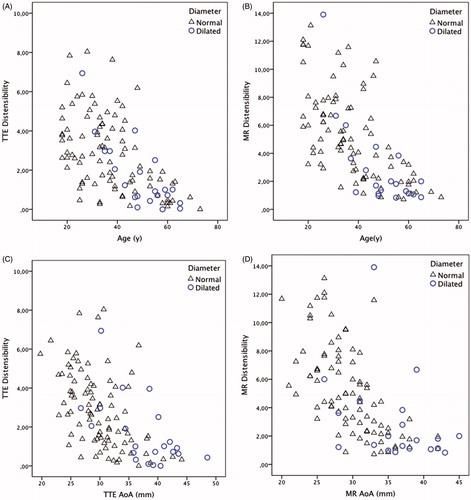
Diameter and distensibility
The diameter of the AoA increased with age, r2 = 0.54. The distensibility of the AoA decreased with increasing age () and with increasing diameter () and this was be seen with both TTE and MRI measurements. The variance of the distensibility seemed to be larger at younger ages but decreased in older individuals and with increasing aortic diameter. This was seen in individuals with a normal aortic diameter as well as in those with a dilated aorta.
PWV
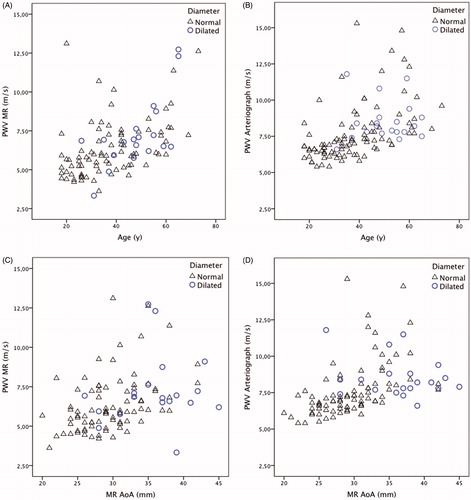
Regional PWV was measured in the thoracic aorta by MRI and global PWV was measured by Arteriograph®. The PWV increased with age () and diameter ().
Differences between individuals with normal and dilated AoAs
Subjects with an AoA diameter >2 SD, when adjusted for height, weight, gender, and age were defined as having a dilated aorta. The individuals with a dilated thoracic aorta were older than individuals without dilatation (49 vs 37 years, p = .001). The individuals with a dilated thoracic aorta showed lower aortic elastic properties than those with normal aortic diameters in all parameters studied (). In the individuals with a dilated thoracic aorta, the distensibility of the AoA measured by TTE (1.01 vs 2.66 mmHg ×10 −3, p = .005) and MRI (1.76 vs 4.53, p = .001) was lower than those with a normal thoracic aorta. Consequently, the PWVs measured by MRI (6.9 vs 5.95 m/s, p = .037) and Arteriograph® (8.2 vs 7.1, p = .001) were higher in individuals with a dilated aorta.
Distensibility
The association between aortic diameter and distensibility measured by MRI differed between individuals with and without a dilated aorta (p = .046), as did the association between age and distensibility (p = .041). In other words, when we adjusted for the differences in age and diameter between the two groups, there was a significantly lower distensibility measured by MRI in individuals with dilated aortas compared with individuals with normal aortic diameters. When distensibility was measured by TTE, there was no difference between groups when adjusted for age and diameter (dilatation*diameter p = .59, dilatation*age p = .09).
PWV
The association between diameter and PWV (p = .045) and between age and PWV (p = .007) measured by MRI differed between individuals with and without dilated aortas. Hence, individuals with a dilated aorta had significantly higher PWVs measured by MRI than individuals without dilatation, even when we adjusted for age and diameter. We also observed a positive association between diameter and PWV measured by Arteriograph® (p = .035). However, the association between age and PWV did not reach statistical significance (p = .73).
Discussion
In this study, we found that (1) there is a strong correlation between AoA diameters measured by TTE and MRI; (2) AoA diameter increases with age; (3) aortic stiffness, measured as distensibility and PWV, increases with age and the diameter of the vessel; and (4) individuals with a dilated AoA have a higher PWV and lower distensibility measured by MRI than individuals with normal aortic diameters, even when adjusted for age and diameter. In cases where images can be obtained, both echocardiography and cardiovascular magnetic resonance provide clinically reliable information on the aortic diameter with very high correlations and minor systematic differences. MRI can be used in screening of family members as a first line investigation because it enables assessment of the diameter of both the ascending and descending aorta and the stiffness of the thoracic aorta. TTE is easy to perform, widely available and can reliably be used in subsequent follow-up.
There are several studies on aortic elastic properties in patients with Marfan syndrome that demonstrated decreased distensibility compared with controls [Citation13–19]. However, we found only one previous publication on aortic stiffness in families with TAAD. Lalande et al. reported that young asymptomatic adults with a MYH11 mutation have an impairment of aortic compliance, which is not detectable when only aortic size is measured [Citation20]. They concluded that aortic compliance measurements might be a part of routine examinations in patients with TAAD and a normal aortic diameter.
A limitation in our study was, that we could not identify all individuals with a dilated aorta as carriers. Also, there are probably several carriers in the non-dilated group. Thus, as we cannot study differences between carriers and non-carriers of the disease-causing variant, conclusions must be drawn with caution. When more information on the genetic variants becomes available, the mechanical properties of the aorta will be even more important to analyze because many carriers may have morphologically normal aortas.
Measuring the diameter of the AoA will probably, in the absence of complimentary diagnostic tools, continue to be the cornerstone for the risk assessment of individuals in families with TAAD. Even if the genetic variant is known, and especially when the genetic variant may cause dissections from near normal aortic diameters, it will be difficult to decide on the timing of prophylactic surgery in individuals with slightly dilated aortas. Thus, efforts to identify useful tools for risk stratification must continue. Measurement of aortic stiffness may give additional information beyond aortic diameter in individuals with TAAD, particularly for those with low distensibility or high PWV at young ages. Registers and longitudinal studies are likely to be useful to collect prospective data on variables beyond aortic dimension. Meanwhile, further research to identify underlying genetic variants is needed so that surveillance, medical therapies and prophylactic interventions can be focused on carriers of FTAAD.
Conclusions
This study shows that TTE and MRI provide clinically reliable information on the ascending aortic diameter with high correlation and only minor systematic differences. MRI can be used for screening the thoracic aorta in FTAAD and TTE can be used in subsequent follow-up of the ascending aorta. Individuals identified to have a dilated thoracic aorta, have lower distensibility and higher PWV than individuals with normal thoracic aortic diameter. Therefore, aortic stiffness might be used as a complementary marker for thoracic aortic disease in families with TAAD.
Acknowledgements
The authors are grateful to the families involved in this study. We also acknowledge Maria Backlund of the Clinical Research Centre of Umeå University as well as Kristofer Ekman and Anna Viklund of the Department of Clinical Physiology of Umeå University.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369.
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926.
- Hannuksela M, Stattin EL, Johansson B, et al. Screening for familial thoracic aortic aneurysms with aortic imaging does not detect all potential carriers of the disease. Aorta. 2015;3:1–8.
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361–367.
- Asch FM, Yuriditsky E, Prakash SK, et al. The need for standardized methods for measuring the aorta: multimodality core lab experience from the GenTAC Registry. JACC Cardiovasc Imaging. 2016;9:219–226.
- Mirea O, Maffessanti F, Gripari P, et al. Effects of aging and body size on proximal and ascending aorta and aortic arch: inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2013;26:419–427.
- Davis AE, Lewandowski AJ, Holloway CJ, et al. Observational study of regional aortic size referenced to body size: production of a cardiovascular magnetic resonance nomogram. J Cardiovasc Magn Reson. 2014;16:9.
- Heiberg E, Sjogren J, Ugander M, et al. Design and validation of segment–freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1.
- Baulmann J, Schillings U, Rickert S, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26:523–528.
- Horvath IG, Nemeth A, Lenkey Z, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075.
- Rajzer MW, Wojciechowska W, Klocek M, et al. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008;26:2001–2007.
- Jatoi NA, Mahmud A, Bennett K, et al. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens. 2009;27:2186–2191.
- Hirata K, Triposkiadis F, Sparks E, et al. The Marfan syndrome: abnormal aortic elastic properties. J Am Coll Cardiol. 1991;18:57–63.
- Savolainen A, Keto P, Hekali P, et al. Aortic distensibility in children with the Marfan syndrome. Am J Cardiol. 1992;70:691–693.
- Sonesson B, Hansen F, Lanne T. Abnormal mechanical properties of the aorta in Marfan's syndrome. Eur J Vasc Surg. 1994;8:595–601.
- Jeremy RW, Huang H, Hwa J, et al. Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol. 1994;74:369–373.
- Adams JN, Brooks M, Redpath TW, et al. Aortic distensibility and stiffness index measured by magnetic resonance imaging in patients with Marfan's syndrome. Br Heart J. 1995;73:265–269.
- Groenink M, de Roos A, Mulder BJ, et al. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82:203–208.
- Westenberg JJ, Scholte AJ, Vaskova Z, et al. Age-related and regional changes of aortic stiffness in the Marfan syndrome: assessment with velocity-encoded MRI. J Magn Reson Imaging. 2011;34:526–531.
- Lalande A, Khau Van Kien P, Walker PM, et al. Compliance and pulse wave velocity assessed by MRI detect early aortic impairment in young patients with mutation of the smooth muscle myosin heavy chain. J Magn Reson Imaging. 2008;28:1180–1187.