Abstract
Objectives. Nesfatin-1 is a novel anorectic neuropeptide with potent metabolic regulatory effects. Nesfatin-1 regulates blood pressure, heart rate, cardiomyocyte metabolism, and permeability. We aimed to evaluate the relationship between carotid artery stenosis (CAS) and nesfatin-1. Design. Three groups were established as patients with no atherosclerotic plaques (n = 60), CAS <60% (n = 60), and CAS ≥60% (n = 60). Then, patients with CAS were divided into 2 subgroups as patients with calcific (n = 67) and non-calcific (n = 53) carotid artery stenosis according to plaque morphological features. Nesfatin-1 levels and baseline data were compared between groups. Results. Serum nesfatin-1 levels were higher in the control group than CAS <60% group (p < .001). Serum nesfatin-1 levels were also higher in CAS <60% group than CAS ≥60% group (p < .001). Multivariable logistic regression analyses demonstrated that serum nesfatin-1 levels were independently associated with CAS. In addition, nesfatin-1 levels were higher in calcific plaques. Conclusions. Nesfatin-1 levels are inversely associated with severtiy of CAS and plaque morphology.
Introduction
Atherosclerosis causes changes ranging from minor wall-thickening to significant luminal stenosis and sometimes occlusion of peripheral, coronary and carotid arteries [Citation1]. Several large epidemiological studies showed associations between carotid artery stenosis, higher risk of ischemic stroke events and coronary artery disease [Citation2]. Plaque morphology and severity of a carotid artery lesion have a significant role in the risk of stroke [Citation2].
Nesfatin-1 is a newly discovered peptide with 82-amino acids [Citation3]. In rats, intracerebroventricular injection of nesfatin-1 causes decreased appetite and then weight loss, while treatment with an antibody, which neutralizes nesfatin-1, leads to increased food intake [Citation3]. Nesfatin-1 was newly found to have cardiovascular actions in the brain by boosting sympathetic nerve activity, thereby increasing mean arterial pressure [Citation4]. Dai et al. found that nesfatin-1 levels were lower in patients presenting with acute myocardial infarction than in normal individuals [Citation5]. In another study we performed, nesfatin-1 levels were lower in patients with slow coronary artery blood flow [Citation6]. Moreover, Ding et al. have found an association between serum nesfatin-1 concentrations and the development and severity of peripheral artery disease in patients with diabetes mellitus [Citation7]. These findings indicate that nesfatin-1 may play a role in atherosclerosis.
The present investigation was designed to assess the association between serum nesfatin-1 and the development and severity of carotid artery stenosis.
Methods
Study population
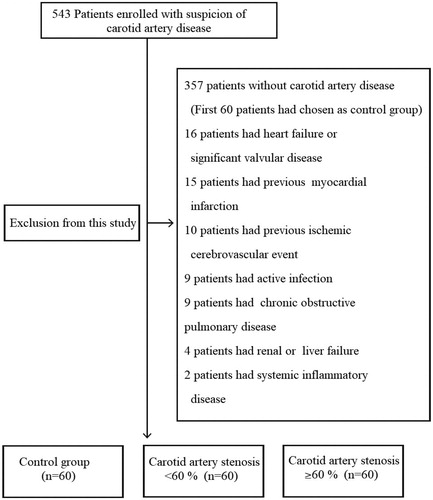
We created three groups with the patients applying to our cardiology outpatient clinic between October 2016 and October 2017. Patients to whom were performed carotid ultrasonography for carotid artery stenosis or to assess cardiovascular risk were included in the study [Citation8]. Each group was planned to consist of 60 individuals. Three groups were established according to the ultrasound results as patients with no atherosclerotic plaques, carotid artery stenosis of less than 60% (CAS <60), and carotid artery stenosis of 60% or more (CAS ≥60). A total of 543 patients were included into the evaluation and the evaluation was completed when each group reached 60 patients. The study was approved by the ethics committee of our institution. Informed consent was taken from all patients. Patients with any carotid artery stenosis detected by carotid ultrasonography examination were also assessed by a multidisciplinary team including a neurologist, cardiologist, radiologist and cardiovascular surgeon and confirmed by this team to be asymptomatic with respect to the carotid artery lesion. Symptomatic patients were excluded from the study when they admitted to our cardiology outpatient clinic and defined as those who experienced an ischemic cerebrovascular event with or without a sequel, a transient ischemic attack or amaurosis fugax within the last 6 months. Nesfatin-1 released from the pituitary is a peptide with a circadian rhythm. There is a potential for the alteration of circadian rhythm due to possible brain injury in patients with symptomatic carotid artery disease and previous studies have revealed some evidence that serum nesfatin-1 levels were affected in the brain injury. We excluded patients with symptomatic carotid artery disease [Citation9]. Because, our main aim was to investigate the relationship between carotid artery atherosclerosis and nesfatin-1. Carotid Doppler ultrasound (CDUS) and then computed tomography angiography (CTA) were performed in all patients. Patients with a history of ischemic or non-ischemic stroke, systemic inflammatory disease, cancer, acute coronary syndrome, previous myocardial infarction, heart failure, significant valvular disease, chronic obstructive pulmonary disease, renal or liver failure, an hematological disease and patients who had an active infection were excluded from the study ().
Definitions
Hypertension was defined as a systolic/diastolic blood pressure of 140/90 mmHg or higher; or patients taking any antihypertensive medication. Diabetes mellitus was defined as a fasting plasma glucose level of ≥7 mmol/L or patients actively using oral antidiabetics and/or insulin. Patients smoking regularly were considered as smokers. Hyperlipidemia was defined as a total cholesterollevel of ≥6.21 mmol/L. Coronary artery disease was defined angiographically as the presence of a plaque in a major coronary artery. Body mass index (BMI) was calculated by dividing the body weight (kg) to the square of the height (m).
Doppler ultrasonography assessments
CDUS examination was performed using Esaote s.p.a MyLabClass C (Florence-Italy) device and a linear arrayed probe that allows selection of frequencies between 3–11 MHZ. Imaging was begun in the transverse plane at the most proximal level obtainable in the common carotid artery (CCA). After the CCA was scanned, the subclavian artery was identified in its long axis by sliding the transducer inferiorly and angling it slightly underneath the clavicle. The transducer was then advanced cephalad toward the carotid bifurcation to identify the internal (ICA) and external carotid arteries. During imaging of the carotid vessels in the transverse orientation, the most stenotic area was carefully evaluated and measured for the percent area/diameter stenosis and/or residual lumen diameter. Then, sagittal images were obtained. Imaging was begun with the proximal CCA. The transducer was aligned so that the CCA appeared in a horizontal position on the screen, without either end angled up or down. This allowed for the best acoustic reflection from the vessel. The transducer was then moved in a cranial fashion to image the bifurcation. Carotid artery stenosis was classified according to NASCET (North American Symptomatic Carotid Endarterectomy Trial) classification. NASCET [Citation10] is a contemporary assessment system, recommended and used for the assessment of carotid artery disease severity [Citation2]. In cases of more than one stenotic lesion or bilateral stenosis, classification was based on the lesion with higher stenosis.
Computed tomography angiography assessments
Carotid artery stenosis was first assessed by CDUS and then by CTA. CTA was performed using a CT device with the Philips Brilliance 64 detector (The Netherlands). After venous access was established through the antecubital vein and 80 mL non-ionic contrast agent was administered at a rate of 4.5 mL/sec, axial-plane CT images of the carotid and cerebral arteries were obtained using the tracking method. Acquired slices were transferred to the work-station (Philips Intellispace Portal) and multi-plane images, maximum intensity projection and volume rendering 3-dimensional images were developed by post-processing the original slices via appropriate software (Advanced Vessel Analysis, Philips). These images were reviewed with respect to vascular plaques and stenosis.
Laboratory measurements
Samples were taken from the antecubital vein at the admission of patients to the hospital. Considering the circadian rhythm of plasma nesfatin-1, samples were taken between 08:00 and 09:00 in the morning. Venous blood was collected from a forearm vein into plain sterile tubes for serum and into ethylenediaminetetraacetic acid (EDTA) tubes for plasma. Basal creatinine level, white blood cell count, platelet count and hemoglobin concentration were measured. The following morning after admission to the hospital, lipid profile and other biochemical parameters were measured using standard techniques. Blood samples to be used for nesfatin-1 measurement were centrifuged immediately and serum samples were stored at -80 °C until the day of analysis. Serum nesfatin-1 levels were measured using a commercial enzyme-linked immunosorbentassay (ELISA) kit (Sensitivity: <37 pmol/L; Assay Range: 114 pmol/L–7342 pmol/L; BosterImmunoleader/USA) as recommended by the manufacturer’s protocol. Nesfatin-1 kits were studied as duplex. Tested coefficient of variation was <10%.
Statistical analysis
Data were analyzed using SPSS version 18.0 statistics package (SPSS Inc., Chicago, IL, USA). Student’s t-test was used for comparison of normally distributed variables and Mann-Whitney U test was used for non-normally distributed variables if 2 groups existed. One-way analysis of variance test was used to compare normally distributed variables between 3 groups. Tukey test was used for post-hoc analysis. Categorical variables were compared by χ2 test or Fisher’s exact test, as appropriate. Pearson’s correlation coefficients were used to assess strength of relationship between continuous variables and Spearman correlation analysis was performed for non-continuous and categorical variables. Major clinical factors and predictors of ≥60% carotid artery stenosis as depicted in were used in univariate and multiple linear regression analysis. In all analyses, p value of <.05 was considered statistically significant and the confidence interval was 95%, continuous variables were reported as mean ± SD and categorical variables were reported as percentages and counts.
Table 1. Baseline characteristics and laboratory parameters of the study groups (n = 180).
Results
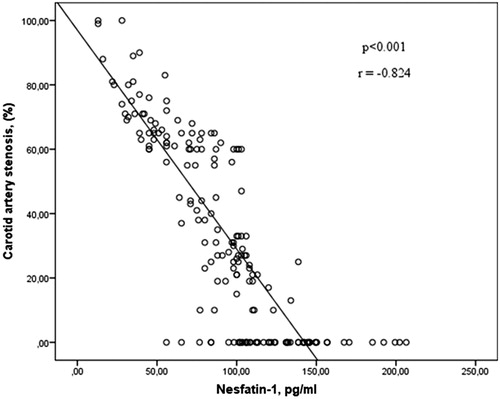
Baseline clinical characteristics and laboratory parameters of the study population are shown in . When the clinical characteristics were evaluated, there was not difference between groups in terms of age, body mass index, sex, diabetes, hypertension, hyperlipidemia, history of coronary artery disease and peripheral artery disease. Smoking rates were found to be higher in the group of patients with carotid artery stenosis of 60% or more compared to other two groups. When carotid plaque characteristics were evaluated, existence CAS ≥60 or CAS <60 contralaterally were found to be higher in the patient group CAS ≥60 than CAS <60. There was not difference in terms of plaque calcification between CAS ≥60 and CAS <60 groups. There was also no difference in terms of biochemical and hematological parameters between the 3 groups except white blood cell and platelet count. Serum nesfatin-1 level was higher in the control group than CAS <60 group and higher in CAS <60 group than CAS ≥60 group. As demonstrated in , there was a negative correlation between serum nesfatin-1 level and rate of carotid artery stenosis. Serum nesfatin levels were detected lower in smokers compared to non-smokers (75.5 ± 35.9 vs 99.5 ± 38.3, p < .001).
We performed univariate and multiple linear regression analysis () for major clinical factors and predictors of ≥60% carotid artery stenosis as depicted in . In univariate regression analysis, serum nesfatin-1 level, smoking, platelet count, and white blood cell count were associated with ≥60% carotid artery stenosis. Lower serum level of nesfatin-1, higher white blood cell count and higher platelet count were detected as independent predictor for ≥60% carotid artery stenosis after multiple linear regression analysis.
Table 2. Multivariate logistic regression analysis showing the predictors for ≥60% carotid artery stenosis.
Patients with carotid artery stenosis were divided into 2 subgroups as patients with calcific (n = 67) and non-calcific (n = 53) carotid artery stenosis according to plaque morphological features. The clinical characteristics, serum nesfatin-1 levels, biochemical and hematological parameters of 2 groups are shown in . No statistically significant difference was determined between these parameters except nesfatin-1 level. Nesfatin-1 levels were higher in the calcific plaque group than in the non-calcific plaque group.
Table 3. Baseline characteristics and laboratory parameters of the patients according to plaque calcification.
Discussion
Our study showed that serum nesfatin-1 levels were lower in patiens with carotid artery disease than those without carotid artery disease. Additionally, this study revealed that nesfatin-1 levels were lower in patients with carotid artery stenosis ≥60% than those with Carotid artery stenosis <60%. Moreover, nesfatin-1 levels had a negative correlation with the rate of carotid artery stenosis. Our study demonstrated that low levels of nesfatin-1 levels was an independent risk marker for carotid artery disease. In addition, nesfatin-1 levels were lower in the noncalcific plaque group than in the calcific plaque group.
Some studies in the last decade revealed that the secretory mediators of adipose tissue such as nesfatin-1 may have a role in the risk of increased cardiovascular disease [Citation11]. Essentially, nesfatin-1 is a satiety hormone and intracerebroventricular injection of this peptide to rats or intraperitoneal application to mice has reduced food intake [Citation3]. Bonnet et al. found an association between inflammation of the brainstem and hypothalamus and activation of neuron expressing nesfatin-1 [Citation12]. Nesfatin-1 administration after head trauma suppressed gene expressions of nuclear factor kappa-B and reduced concentrations of inflammation markers like tumor necrosis factor-alpha, interleukin-1b and interleukin-6 in traumatic brain tissues of rat [Citation9]. Inflammation plays a role in the every stage of atherosclerosis, including its development, progression and rupture of the atherosclerotic plaque [Citation13]. Likewise, inflammation plays an important role in the pathophysiology of carotid atherosclerosis [Citation14]. The other two important factors playing an important role in the pathophysiology of carotid artery disease are oxidative stress and endothelial dysfunction [Citation15]. Ayada et al. demonstrated that chronic peripheral infusion of nesfatin-1 lowered synthesis of endothelial nitric oxide especially in chronically restraint stressed rat [Citation16].
Non-calcified carotid plaques are more prone to unstablity than calcified plaques, and that’s why, they are associated with a higher risk of rupture, thromboembolism and stroke [Citation17]. Van Lammeren et al. demonstrated that patients with asymptomatic carotid atherosclerotic plaques have relatively more stable plaque characteristics with a higher plaque smooth muscle cell content, a higher proportion of heavily calcified plaques, and less frequent intraplaque haemorrhages compared to patients with ipsilateral cerebrovascular events [Citation17]. Adiponectins, which play a role in adipose tissue metobolism such as nesfatin-1, has the potential to affect the morphology of atherosclerotic plaque [Citation18,Citation19]. Nesfatin-1 is in close relationship with inflammation and inflammation is closely associated with instability and morphology of carotid artery plaque [Citation14].
Neuropeptides such as nesfatin-1, ghrelin, leptin, and adiponectin play significant roles in body mass index, dietary habits, and body metabolism and effect, body elimination and biochemical properties of these neuropeptides are similar [Citation20,Citation21]. Most of them have a role in the pathophysiology of atherosclerosis [Citation22,Citation23]. Adiponectin and ghrelin have protective properties on endothelial function, while leptin has effects leading to endothelial dysfunction [Citation24,Citation25]. Smoking has the potential to affect the metabolism of many neuropeptides. Ghrelin levels were found to be higher in smokers than in non-smokers [Citation26]. In another study, adiponectin and leptin levels were detected elevated in individulas who quit smoking [Citation27]. Nesfatin-1 levels were lower in smokers (p < .001) and smoking was higher in patients with severe carotid artery disease in our study (p = .017). In the light of these findings, smoking has the potential to interact with the metabolism of nesfatin-1.
Dai et al found that patients with acute coronary syndrome had lower nesfatin-1 levels (2.89 ± 0.25 nmol/L) than patients with stable angina pectoris (3.12 ± 0.60 nmol/L) and patients with stable angina pectoris had lower nesfatin-1 levels than those with normal coronary artery disease (3.47 ± 1.24 nmol/L). In addition, they found a negative correlation between nesfatin-1 levels and the Gensini score indicating coronary artery disease severity. As compatible to our hypothesis, negative correlation of nesfatin 1 level with neutrophil percentage and CRP was found in the study [Citation5]. Furthermore, Ding et al. discovered an association between serum nesfatin-1 concentrations and the development and severity of peripheral arterial disease in patients with diabetes mellitus [Citation7]. In our study, carotid artery stenosis severity and nesfatin-1 levels were also found to be closely associated with each other. Considering these studies, nesfatin-1 has the potential to play a role in the pathophysiology of atherosclerosis. More advanced studies evaluating the relationship between nesfatin-1 and atherosclerosis may be helpful in elucidating the pathophysiology of atherosclerosis.
Limitations of the study
The present study is a cross-sectional study with relatively small sample size. We don’t have follow up on major adverse cardiovascular events data. So, our results should be verified in the multi-center prospective longitudinal studies with larger sample size. In addition, there is no evaluation system, which determines the diffuseness and severity of carotid artery disease, like SYNTAX score, which is the most commonly used scoring system that has been tested in the study of SYNTAX [Citation28]. The limitations of this study should be considered while interpreting the results.
Conclusions
Nesfatin-1 levels showed a negative correlation with the rate of carotid artery stenosis in our study. Nesfatin-1 levels are closely associated with plaque morphology. Nesfatin-1 has the potential to play a role in the pathophysiology of atherosclerosis. Further studies are required to determine the relation between carotid artery disease and nesfatin-1.
Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695.
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC), and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816.
- Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 2006;443:709–712.
- Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–R336.
- Dai H, Li X, He T, et al. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167–171.
- Kuyumcu MS, Kuyumcu A, Yayla C, et al. Nesfatin-1 levels in patients with slow coronary flow. Kardiol Pol. 2018;76:401–405.
- Ding S, Qu W, Dang S, et al. Serum nesfatin-1 is reduced in type 2 diabetes mellitus patients with peripheral arterial disease. Med Sci Monit. 2015;21:91.
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–274.
- Ferguson GG, Eliasziw M, Barr HWK, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758.
- Feijoo-Bandin S, Rodriguez-Penas D, Garcia-Rua V, et al. Nesfatin-1: a new energy-regulating peptide with pleiotropic functions. Implications at cardiovascular level. Endocrine. 2016;52:11–29.
- Bonnet MS, Pecchi E, Trouslard J, et al. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27.
- Tang C-H, Fu X-J, Xu X-L, et al. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides. 2012;36:39–45.
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874.
- Jander S, Sitzer M, Schumann R, et al. Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke. 1998;29:1625–1630.
- Signorelli SS, Neri S, Di Pino L, et al. Oxidative stress and endothelial damage in patients with asymptomatic carotid atherosclerosis. Clin Exp Med. 2001;1:9–12.
- Ayada C, Turgut G, Turgut S, et al. The effect of chronic peripheral nesfatin-1 application on blood pressure in normal and chronic restraint stressed rats: related with circulating level of blood pressure regulators. GPB. 2015;34:81–88.
- van Lammeren GW, den Hartog AG, Pasterkamp G, et al. Asymptomatic carotid artery stenosis: identification of subgroups with different underlying plaque characteristics. Eur J Vasc Endovasc Surg. 2012;43:632–636.
- Takeuchi S, Wada K, Uozumi Y, et al. Adiponectin receptor 1 expression is associated with carotid plaque stability. Neurol India. 2013;61:249–253.
- Musialek P, Tracz W, Tekieli L, et al. Multimarker approach in discriminating patients with symptomatic and asymptomatic atherosclerotic carotid artery stenosis. J Clin Neurol. 2013;9:165–175.
- Ozkan Y, Timurkan ES, Aydin S, et al. Acylated and desacylated ghrelin, preptin, leptin, and nesfatin-1 peptide changes related to the body mass index. Int J Endocrinol. 2013;2013:236085.
- Hallberg M. Neuropeptides: metabolism to bioactive fragments and the pharmacology of their receptors. Med Res Rev. 2015;35:464–519.
- Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clin Chim Acta. 2004;344:1–12.
- Ukkola O, Poykko S, Paivansalo M, et al. Interactions between ghrelin, leptin and IGF-I affect metabolic syndrome and early atherosclerosis. Ann Med. 2008;40:465–473.
- Koleva DI, Orbetzova MM, Nikolova JG, et al. Pathophysiological role of adiponectin, leptin and asymmetric dimethylarginine in the process of atherosclerosis. Folia Med (Plovdiv). 2016;58:234–240.
- Virdis A, Duranti E, Colucci R, et al. Ghrelin restores nitric oxide availability in resistance circulation of essential hypertensive patients: role of NAD(P)H oxidase. Eur Heart J. 2015;36:3023–3030.
- Koopmann A, Bez J, Lemenager T, et al. Effects of cigarette smoking on plasma concentration of the appetite-regulating peptide ghrelin. Ann Nutr Metab. 2015;66:155–161.
- Toffolo MCF, Gomes ADS, Van Keulen HV, et al. Alteration of inflammatory adipokines after four months of smoking abstinence in multidisciplinary intervention program. Nutr Hosp. 2018;35:434–441.
- Serruys PW, Morice M-C, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972.