Abstract
Background. Left ventricular (LV) remodeling and clinical response to cardiac resynchronization therapy (CRT) is inversely related to electrical dyssynchrony, measured as LV lead electrical delay (QLV). Presence of atrial or ventricular arrhythmia is correlated with worsening heart failure and LV remodeling. Objective. We sought to assess the association of QLV with arrhythmic events in CRT recipients. Methods. We identified patients implanted with a CRT device at our center. QLV interval was measured and corrected for baseline QRS (cQLV). We performed multivariable Logistic regression to assess the effect of cQLV on the occurrence of atrial/ventricular arrhythmic events. Results. Sixty-nine patients were included in analyses. The cQLV was significantly shorter in patients with atria tachycardia/supraventricular tachycardia (AT/SVT) events compared to patients without AT/SVT events (43.4 ± 22% vs. 60.3 ± 26.7%, p = .006). In contrast, no significant difference in cQLV was observed between patients with and without ventricular tachycardia/fibrillation (VT/VF) events (46.2 ± 25.4% vs. 56 ± 25.7%, p = .13). cQLV was significantly shorter in patients with new onset AT/SVT events compared to those without (38.3 ± 22.2% vs. 55.7 ± 25.7%, p = .028). In contrast, no significant difference in cQLV was observed between patients with and without new onset VT/VF events (44.2 ± 25.2% vs. 56.3 ± 25.5%, p = .069). Following adjusted analyses, cQLV was a significant predictor of AT/SVT, but not for VT/VF. Conclusion. cQLV is a simple measure that can identify a vulnerable cohort of CRT patients at increased risk for atrial tachyarrhythmias, and hence can predict reverse remodeling and clinical response to CRT treatment.
Introduction
Patients with advanced heart failure (HF), left ventricular (LV) systolic dysfunction and wide QRS complex show significant reduction in hospitalization and mortality with the use of cardiac resynchronization therapy (CRT) [Citation1]. CRT has also been shown to improve the quality of life, exercise capacity and functional status in this group of patients [Citation2]. American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend CRT for the treatment of class III or ambulatory class IV HF symptoms with optimal recommended medical therapy in patients with severe systolic HF who have LV ejection fraction less than or equal to 35%, a QRS duration (QRSd) greater than or equal to 0.12 seconds, and sinus rhythm [Citation3].
Patients with left bundle branch block (LBBB) undergoing CRT have better outcomes, including a reduction in mortality and HF events as compared to CRT patients with a non- LBBB pattern [Citation4]. However, LBBB and QRSd do not predict CRT response after adjusting for LV lead electrical delay (QLV) [Citation5]. This interval is defined as the time from the onset of the QRS of the surface ECG to the first large positive or negative peak of the LV electrogram (EGM) during a cardiac cycle. The QLV reflects the degree of delayed LV activation at the pacing site and may predict hemodynamic response and long-term clinical outcomes [Citation6]. In fact, this measure of electrical dyssynchrony is strongly associated with reverse remodeling of the heart with CRT [Citation7].
One other method to measure delayed LV activation is the Corrected QLV (cQLV). The cQLV is the ratio of QLV over the QRSd. It represents a clear specific index of the electrical delay where QLV interval is adjusted to each QRSd, and thus eliminating a potential impact of the QRSd duration on the predictive value of the local electrical delay [Citation8,Citation9]. Additionally, QLV interval correlates with QRSd and both variables synergistically predict short-term CRT response, however QRSd prolongation is considered as a risk factor for cardiac mortality in HF patients, so by using only QLV interval instead of cQLV, the risk-predictive power may be attenuated [Citation8].
It has been shown that longer QLV is associated with better CRT response [Citation10]; however, electrical dyssynchrony may predispose the myocardium to arrhythmias [Citation6]. These arrhythmias may serve as a marker for poor CRT response. To investigate this further, we evaluated the relationship between cQLV and arrhythmic events, and determined whether cQLV can predict e77143e77143arrhythmia, and hence can be used as an early marker to predict clinical response in patients with CRT.
Material and methods
Study design and population
This is a single center retrospective cohort study. The study was approved by the ethics committee of the hospital (institutional review board). Subjects included in the study were patients on cardiac resynchronization implantable cardioverter defibrillator (CRT-D) for a minimum duration of one year and actively followed by the implantable cardioverter-defibrillator (ICD) Program at Hartford Hospital. All patients were receiving optimal medical therapy for HF. The exclusion criteria were (i) pacemaker-dependent subjects with complete heart block and no underlying cardiac rhythm, (ii) CRT-D patients demonstrating less than 90% pacing (iii) CRT-D patients with underlying atrial fibrillation (AF).
At routine ICD clinic visits, patients meeting the entry criteria were evaluated.
All EGMs in the CRT interrogation reports were reviewed by 2 board certified electrophysiologists for the occurrence of arrhythmias and far-field sensing. Endless loop tachycardia or repetitive non-reentrant ventriculoatrial synchrony were excluded by visual review by both the electrophysiologists. The threshold for atrial sensing was 0.5 mv. AF events were included in atria tachycardia/supraventricular tachycardia (AT/SVT) events, and was defined as at least 1 or more episodes of AT/SVT lasting at for at least 30 seconds, detected clinically by electrocardiography or by identification of atrial high–rate event on device counters, at any time after device implantation. Ventricular arrhythmia (VA) was defined as 1 or more episodes of VT lasting at least 30 seconds, or the occurrence of ventricular fibrillation (VF). Baseline demographics and ECG data before the implantation procedure, including the pre-implantation QRS width, PR interval, QRS axis, and QRS bundle branch morphological features were recorded. An EGM with simultaneous recording of surface ECG lead II, LV EGM and right ventricular BiV-ICD leads was performed at a sweep speed of 50 mm/s in sinus or atrial paced rhythm without ventricular pacing.
The QLV interval was measured in sinus or atrial paced rhythm without ventricular pacing from the onset of the QRS from the surface ECG to the first positive or negative peak of the LV EGM with the resolution of 10 ms during a cardiac cycle by an investigator blinded to patient history. The amplitude of the first large peak needed to be >50% of the amplitude of the largest peak in the same cardiac cycle. The QLV was corrected for baseline QRS and the cQLV measured as a percentage of QRS was recorded.
Outcome measures
Atrial and ventricular arrhythmic events and the anti-arrhythmic therapies of anti-tachycardia pacing or shocks delivered by the ICD were recorded. Patients that developed arrhythmic events were compared to CRT-D patients without arrhythmic events. The association between arrhythmic outcomes of AT/SVT and VT/VF with cQLV was determined
Statistical analysis
Descriptive statistics were used to describe baseline characteristics of our sample. Continuous variable were presented as mean ± SD; differences in these variables between patients who did and those who did not experience the outcome(s) were assessed using Student’s t-test. Categorical data were summarized as frequencies (percentages); differences in these variables between patients who did and those who did not experience the outcome(s) were assessed using Chi-squared or Fisher’s Exact Tests, as appropriate. We fit unadjusted logistic regression models to assess the association of baseline characteristics and cQLV individually with the occurrence of ≥1 arrhythmic event. We then constructed a multivariable logistic regression model to quantify the relationship between cQLV and the occurrence of arrhythmic events, while holding other baseline/clinical variables constant. This model was constructed based on clinical judgement; the covariates were included to eliminate potential confounding, as the selected covariates had been previously associated with arrhythmic events. Tests were performed assuming a Type I error rate of 5% and a 2-sided alternative hypothesis. All analyses were performed using SPSS statistical software.
Results
Patient characteristics
There were 69 patients who met criteria for this study. A summary of baseline clinical data is in . The mean age of the study cohort was 72 ± 10 years with a mean cQLV of 52.4% ± 25.8%.
Table 1. Patient characteristics (n = 69).
AT/SVT episodes occurred in 46.4% (32/69) of subjects and VT/VF were observed in 36.2% (25/69) of subjects over a mean follow up period of 43.8 months. The incidence of (new onset) AT/AF and VT/VF was 18.8% (n = 13) and 31.9% (n = 22), respectively.
Corrected lead electrical delay
The cQLV was significantly shorter in patients with AT/SVT events compared to patients without (43.4 ± 22% vs. 60.3 ± 26.7%, p = .006) (). No significant difference in cQLV was observed between patients with and without VT/VF events (46.2 ± 25.4% vs. 56 ± 25.7%, p = .133) (). Among patients with new onset of arrhythmias after CRT-D implantation (), cQLV was significantly shorter in patients with new onset AT/SVT events (38.3 ± 22.2% vs. 55.7 ± 25.7%, p = .028). Similarly, no significant difference in cQLV was observed between patients with and without new onset VT/VF events (44.2 ± 25.2% vs. 56.3 ± 25.5%, p = .069).
Figure 1. Association of corrected LV lead electrical delay (cQLV) with arrhythmic events among CRT recipients. Figure 1A: cQLV in patients with atria tachycardia/supraventricular tachycardia (AT/SVT) events compared to patients without AT/SVT events (43.4 ± 22% vs. 60.3 ± 26.7%, p = .006.); Figure 1B: cQLV in patients with ventricular tachycardia/fibrillation (VT/VF) events compared to patients without VT/VF events (46.2 ± 25.4% vs. 56 ± 25.7%, p = .13).
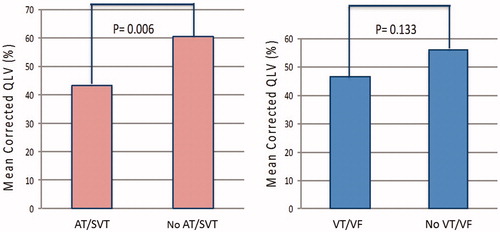
Figure 2. Association of corrected LV lead electrical delay (cQLV) with new onset arrhythmic events among CRT recipients. Figure 2A: cQLV in patients with new onset atria tachycardia/supraventricular tachycardia (AT/SVT) events compared to patients without new onset AT/SVT events (38.3 ± 22.2% vs. 55.7 ± 25.7%, p = .028); Figure 2B: cQLV in patients with new onset ventricular tachycardia/fibrillation (VT/VF) events compared to patients without new onset VT/VF events (44.2 ± 25.2% vs. 56.3 ± 25.5%, p = .069).
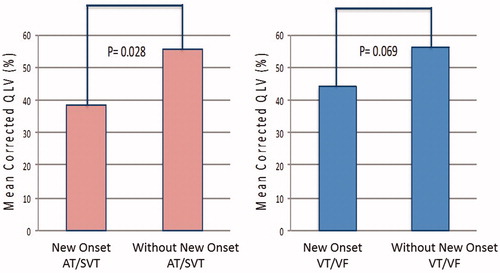
Table 2. Comparison of demographic and clinical characteristics of patients with and without arrhythmic events.
Table 3. Odds ratios for arrhythmic events.
Logistic regression
A 1-unit increase in cQLV was associated with a 3% decrease in risk for AT/SVT (adjusted odds ratio: 0.97, 95% confidence interval: 0.95–0.996, p = .019). Similarly, a 1-unit increase in cQLV was associated with a 4% decrease in risk for new onset AT/SVT (adjusted odds ratio: 0.96, 95% confidence interval: 0.93–0.998, p = .019) (). Conversely, cQLV was not associated with a change in risk for VT/VF.
Discussion
In this study of 69 patients with CRT-D, we determined that cQLV was predictive of AT/SVT arrhythmic events. In particular, longer cQLV at the stimulation site was associated with lower risk of AT/SVT. A trend towards higher incidence of VT/VF in subjects with shorter cQLV was also observed in the univariate analysis; however, it was not a statistically significant predictor of VT/VF.
The importance of QLV as a determinant of CRT response has been demonstrated in various studies. The study by Gold et al. demonstrated better CRT response by reverse LV remodeling with longer QLV intervals [Citation5]. Singh et al. demonstrated that the percentage of LV delay as a function of QRSd predicts hemodynamic and long-term clinical outcomes following CRT implantation [Citation6]. CRT restores the electrical synchrony by pre-exciting the delayed LV area to achieve more synchronous electrical activation and contraction in the LV [Citation11]. Myocardium with dyssynchronous electrical activity is prone to development of arrhythmias. Analysis of the Multicenter Automatic Defibrillator Implantation Trial- Cardiac Resynchronization Therapy (MADIT-CRT) study population by Zareba et al. demonstrated significantly reduced incidence of VT/VF events in LBBB patients treated with CRT [Citation4]. Besides VA, AT including atrial flutter and AF are frequently observed in patients with HF and its presence has shown to correlate with worsening of the symptoms [Citation12]. A worse prognosis and increased mortality are observed in HF patients who develop AF [Citation13]. Studies have shown that CRT induces favorable reverse remodeling effects on the left atrium (LA) that may reduce the risk of developing atrial arrhythmia in HF patients [Citation14]. Brenyo et al. studied the risk of atrial tachyarrhythmias in patients enrolled in the MADIT-CRT trial [Citation15]. The study showed that in patients with mild HF, CRT was associated with marked reverse remodeling effects on the LA. A reduction in the risk of AT/AF was observed in the patients with a reduction in the LA volume. Development of AT after the implantation of CRT was associated with a significant increase in morbidity and mortality. This suggests that development of atrial tachyarrhythmias is a negative indicator of reverse remodeling. Our study has demonstrated that shorter cQLV predicts atrial arrhythmias, perhaps this can be secondary to the non-physiological atrioventricular (AV) intervals and forced ventricular pacing induced by CRT. Forcing a short AV interval can result in delay of completion of LA mechanical systole after mitral valve closure, increasing LA pressure. Since CRT patients are more prone to atrial arrhythmic event, and its occurrence is associated with worsening clinical outcome, early detection of arrhythmic event and perhaps controlling it can possibly lead to better outcome and less morbidity in CRT patients. Our study suggests that cQLV can predict atrial arrhythmias in HF patients on CRT and may serve as a surrogate marker for predicting atrial reverse remodeling and clinical response to CRT treatment in this patient population.
Kutyifa et al. also evaluated the relationship between LV dyssynchrony and the risk of VT or VF in patients enrolled in the MADIT-CRT trial [Citation16]. Improvement of LV dyssynchrony after CRT implantation was associated with significant reduction of VA in patients with LBBB. In a small study of patients with non-apical lead by Friedman et al., an LV lead delay of <50% native QRS was observed to be an independent predictor of VA [Citation17]. Patients with an LV lead delay ≥50% native QRS were at lower risk for first incident and recurrent VA. Our study demonstrated a similar trend in unadjusted analyses of patients with longer cQLV, suggesting higher synchrony and better CRT response had a trend towards lower frequency of VT/VF events; however, the effect lost statistical significance in the adjusted analysis.
Study limitations
A primary limitation of this study is the small sample size. As a single center, retrospective study, the recruitment of subjects was limited to the patients visiting the ICD clinic for follow up checks. Perhaps a larger cohort may have yielded sufficient statistical power statistically significant difference in the VT/VF events with a change in the cQLV. Data regarding the arrhythmic events was derived from the device interrogations performed during clinic visits after CRT-D implantation. Thus, lower rate of AT episodes was not standardized and included in the analysis. The settings for ventricular tachycardia zone was mostly >170 beats/min, but may have been changed in some patients depending upon the clinical requirements. It is unknown if there could be a minor change in the QLV duration over a period of time. Given that the study was retrospective, LA and LV dimensions in echocardiogram data for all study cohort were not available, this limited our ability to clearly comment on the relationship between remodeling and cQLV. The QLV was not measured at the time of implantation of the CRT-D, rather it was measured from the electrogram during follow up clinic visit of the patients.
Conclusion
In CRT Patients, development of atrial tachyarrhythmia and electrical dyssynchrony, (represented by QLV or cQLV), were shown in previous studies to herald poor outcomes in HF patients on CRT and to be inversely related to LV remodeling and clinical response to CRT treatment. cQLV can predict atrial arrhythmias in HF patients on CRT, it may serve as a surrogate marker for predicting reverse remodeling and clinical response to CRT treatment in this patient population. By attempting to keep the QLV longer during the time of CRT implantation, it may translate into a reduction of the risk of future atrial tachyarrhythmias, and subsequent mortality, among patients with HF on CRT.
Disclosure
No potential conflict of interest was reported by the authors.
References
- Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150.
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:350–308.
- Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123:1061–1072.
- Gold MR, Birgersdotter-Green U, Singh JP, et al. The relationship between ventricular electrical delay and left ventricular remodeling with cardiac resynchronization therapy. Eur Heart J. 2011;32:2516–2524.
- Singh JP, Fan D, Heist KE, et al. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006;3:1285–1292.
- Merchant FM, Heist EK, Nandigam KV, et al. Interlead distance and left ventricular lead electrical delay predict reverse remodeling during cardiac resynchronization therapy. Pace. 2010; 33:575–582.
- Roubicek T, Wichterle D, Kucera P, et al. Left ventricular lead electrical delay is a predictor of mortality in patients with cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2015;8:1113–1121.
- Fatemi M, Le Gal G, Blanc JJ, et al. The use of epicardial electrogram as a simple guide to select the optimal site of left ventricular pacing in cardiac resynchronization therapy. Cardiol Res Pract. 2011;2011:1.
- Kandala J, Upadhyay GA, Altman RK, et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J. 2013;34:2252–2262.
- Yu Y, Kramer A, Spinelli J, et al. Biventricular mechanical asynchrony predicts hemodynamic effect of uni- and biventricular pacing. Am J Physiol Heart Circ Physiol. 2003;285:H2788–H2796.
- Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2–8.
- Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703.
- Fung JW, Yip GW, Zhang Q, et al. Improvement of left atrial function is associated with lower incidence of atrial fibrillation and mortality after cardiac resynchronization therapy. Heart Rhythm. 2008;5:780–786.
- Brenyo A, Link MS, Barsheshet A, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;58:1682–1689.
- Kutyifa V, Pouleur AC, Knappe D, et al. Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6:432–444.
- Friedman DJ, Upadhyay GA, Altman RK, et al. The anatomic and electrical location of the left ventricular lead predicts ventricular arrhythmia in cardiac resynchronization therapy. Heart Rhythm. 2013;10:668–675.
