Abstract
Rationale. Atrial fibrillation is associated with increased mortality as well as morbidity. There is strong evidence for an association between atrial fibrillation and sleep apnea. It is not known whether treatment of sleep apnea with continuous positive airway pressure (CPAP) will reduce the burden of atrial fibrillation.
Objective. The Treatment of Sleep Apnea in Patients with Paroxysmal Atrial Fibrillation study will investigate the effects of CPAP in patients with paroxysmal atrial fibrillation and sleep apnea.
Design: The trial has a dual center, randomized, controlled, open-label, parallel design.
Methods. Two centers will enroll a total of 100 patients with both paroxysmal atrial fibrillation and sleep apnea (apnea-hypopnea index [AHI] ≥ 15 events/h) who are scheduled for catheter ablation. Patients will be randomized in a 1:1 ratio to CPAP or control group (50 patients in each arm). The effects of CPAP treatment on atrial fibrillation will be determined using an implanted loop recorder (Reveal LINQ™, Medtronic) that detects all arrhythmia episodes. The primary endpoint is a reduction of the total burden of atrial fibrillation in the intervention group, after 5 months’ follow-up (preablation). Reduction in the arrhythmia recurrence rate after ablation is the main secondary endpoint. All patients will be followed up for 12 months after ablation.
Conclusion. This study is the first randomized controlled trial that will provide data on the effects of CPAP therapy in patients with paroxysmal atrial fibrillation and sleep apnea. The results are expected to improve our understanding of the interaction between paroxysmal atrial fibrillation and sleep apnea.
Trial registration: ClinicalTrials.gov identifier: NCT02727192.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disturbance in adults. The prevalence of AF is around 2.5% in individuals aged >40 years, increasing to >10% in those aged >80 years [Citation1],with rates expected to rise significantly in coming decades. The presence of AF significantly increases mortality as well as morbidity, particularly the risk of cerebrovascular accidents. Catheter ablation of AF is recognized as a valid treatment option, with the main indication being elimination of symptomatic AF when pharmacological therapy is less effective or has failed. However, the recurrence rate after ablation is high and a substantial proportion of patients require sustained pharmacological treatment as well as lifelong anticoagulation.
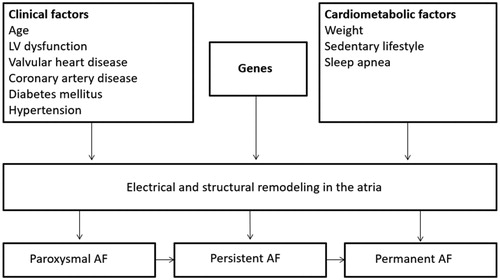
The reasons for the limited success of both pharmacological and ablation therapy are unclear, but may be due to the heterogeneous and complex pathophysiology of AF. Recent data have suggested that, in addition to clinical risk factors, several cardiometabolic factors such as sleep apnea (SA), obesity and sedentary lifestyle are associated with adverse electrical and structural heart remodelling (). This is supported by data from randomized controlled trials showing that aerobic interval training or combined risk factor interventions reduce the arrhythmic burden in AF patients and is associated with significant improvements in AF symptoms, and quality of life [Citation2–4].
Figure 1. Risk factors and triggers of atrial fibrillation. AF, atrial fibrillation; LV, left ventricular.
SA is a common chronic disorder, also in patients with cardiovascular (CV) disorders [Citation5–7]. The reduction of the airflow in obstructive sleep apnea (OSA), characterized by nocturnal oxygen desaturation, is associated with a number of physiological and biochemical alternations, including sympathetic activation [Citation8,Citation9], oxidative stress [Citation10,Citation11] and systemic inflammation [Citation12–14].
There is now strong evidence for an association between AF and SA [Citation15–17], with reported SA prevalence rates of 40–75% in patients with AF [Citation18]. The type of SA seen in patients with AF is mainly OSA, although central sleep apnea (CSA) also can be observed. The prevalence of OSA in AF patients is several times higher than in the general population, which in Norway is estimated to be 16% (apnea-hypopnea index [AHI] ≥ 5/h) and 8% (AHI ≥15/h) [Citation19]. Studies have also demonstrated that OSA diagnosis and severity are independently associated with incident AF and recurrence of AF after ablation [Citation20]. This suggests that treatment of OSA might reduce the clinical burden of AF. However, the impact of treating OSA on the incidence and burden of AF has not yet been well established [Citation21–23]. The randomized controlled trial “CPAP Treatment of Sleep Apnea in Patients with Paroxysmal Atrial Fibrillation” (acronym: A3) was therefore designed to investigate the effects of CPAP treatment in patients with paroxysmal AF (PAF) and SA.
Methods
Study design
The A3 study is a randomized, controlled, open-label, parallel group trial being conducted at two centers in Norway, to determine wether treatment of SA with CPAP will reduce the total burden of AF. The trial has been registered at clinicaltrials.gov (NCT02727192, www.clinicaltrials.gov). It is planned to include a total of 100 patients with both PAF and SA (AHI) ≥15/h) who are scheduled for catheter ablation. Patients will be randomized to CPAP treatment or control. Randomization and allocation to treatment or control arm is being done using the online platform ViedocTM (PCG Solutions, Uppsala, Sweden) and will take place one month after implantation of a subcutaneous arrhythmia detector (RevealLINQ™, loop recorder, Medtronic). The randomization list will be generated using Stata (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) with a 1:1 allocation using random block sizes of 4, 6, 8, and 10. Patients will be assigned a unique patient identification number. In addition, 10 patients with no SA (AHI <5/h) and 10 patients with mild SA (AHI 5–15/h) will be studied for comparison. Arrhythmia burden will be monitored both by intermittent interrogation of the loop recorders, and by continuous monitoring via the Medtronic CareLink® Home Monitor. The monitor allows patients to send data from their loop recorder via the mobile network directly to the clinic. This allows a remote follow-up of the patients. Access to diagnostic device reports and notifications is available 24/7 on the secure CareLink website.
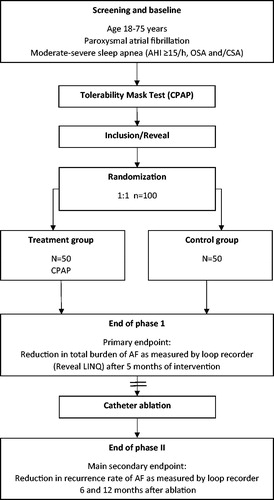
Inclusion and exclusion criteria are presented in . summarizes the endpoints in the A3 study, and outlines the study design.
Figure 2. Study flow-chart. AF: atrial fibrillation; AHI: apnea-hypopnea index; CSA: central sleep apnea; CPAP: continuous positive airway pressure; OSA: obstructive sleep apnea.
The Trial design was approved by the local ethics committees South-East Regional Ethics Committee (REK, ID: 2015/436) and the ethic and data inspector of Oslo University Hospital (personvernombud). The trial is being conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provide written informed consent to participate in the study.
Patient population
Eligible patients are those with PAF scheduled to undergo first or second catheter ablation. Specific inclusion and exclusion criteria are presented in . Patients meeting these criteria will be invited to undergo 2 nights of polygraphy (PG) (T3 Nox, ResMed) at home. Those with an AHI of ≥15/h (OSA and/or CSA) will be invited to participate if they tolerate CPAP therapy. Tolerance of CPAP therapy will be determined using a 1-week trial of CPAP, using standard settings and pressure levels (4–20 cm H2O). Adjustments are based on close follow-up by telephone and device data (obtained using continuous home monitoring Airview™; ResMed). Compliance is defined as device usage for >4 h per night every night for 7 nights.
Table 1. A3 Study inclusion and exclusion criteria.
Table 2. Summary of A3 study endpoints.
Polygraphy assessment
All PG recordings will be analyzed at a core sleep lab by an independent specialist who is unaware of treatment allocation. Events will be scored using standard American Academy of Sleep Medicine (AASM) [Citation24] definitions: Apnea is defined as a ≥ 90% drop in the flow signal for ≥10 seconds and classified as either obstructive (if accompanied by typical thoracic-abdominal breathing effort) or central (if there is no effort). Hypopnea is defined as a ≥ 30% reduction in airflow followed by a ≥ 3% fall in oxygen saturation. The hypopneas are classified as obstructive if there was observed snoring, increased inspiratory flattening of the nasal pressure or paradoxical thoracoabdominal movements. The AHI is calculated as the number of episodes of apnea and hypopnea per hour of recording time.
Loop recorder implantation
A loop recorder device (Reveal LINQ™, Medtronic) will be implanted subcutaneously, in a left parasternal position. This device continuously records a single-lead ECG and has an algorithm designed to detect AF by looking at the irregularity and incoherence of R-R intervals. A previous study has demonstrated that the device can correctly identify AF in 96.1% of patients and correctly exclude AF in 97.4% of patients [Citation25].
Interventions
Patients in the therapy group will be given positive airway pressure (PAP) based on the most appropriate treatment identified during tolerance testing. CPAP [AirSense 10 AutoSet, ResMed] for predominant OSA or adaptive servo-ventilation (ASV) [PaceWave, Auto Set CS, ResMed] for patients with a central apnea index [cAI] > 10/h). PAP therapy will be monitored remotely using AirView™ (ResMed) and in addition data will be downloaded from the device every three months. Patients in the control group are not given PAP therapy of any sort, but will be provided with education on healthy lifestyle and sleep.
Assessments
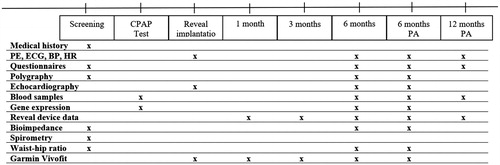
Procedures and measurements are presented in the timeline .
Figure 3. Study procedures. BP: blood pressure; CPAP: Continuous positive airway pressure; ECG: electrocardiogram; HR: heart rate; PA: post-ablation; PE: physicial examination.
Quality of life is assessed using the Short Form-36 (SF-36) [Citation26] and Functional Outcomes of Sleep Questionnaire (FOSQ) [Citation27] The Atrial Fibrillation Severity Scale (AFSS) questionnaire will be used to capture subjective and objective ratings of AF-related symptoms and AF disease burden, including frequency, duration, and severity of episodes. Daytime sleepiness, sleep quality and symptoms of SA will be measured using the Epworth Sleepiness Scale (ESS) [Citation28], the Berlin Questionnaire and the Stop-Bang Questionnaire.
Bioelectric Impedance weight (Tanita BC-545N) is used to calculate body composition, and will provide a quick overview of the water and fat percentage in the body, predicted muscle and bone mass, visceral fat, basal metabolic rate, metabolic age and physique rating.
Echocardiography
Echocardiography will be used to determine the left ventricular ejection fraction, valvular function and left atrial size. In addition to standard recording, special emphasis on the left atrium with strain and 3D echocardiography will be performed. A transesophageal echocardiography will be performed in all patients prior to catheter ablation.
Gene expression analysis
White blood cells will be analyzed using real-time quantitative polymerase chain reaction (RT-PCR) and ribonuclease protection assay. The aim is to investigate whether PAF is associated with specific gene expression patterns, examine the influence of SA treatment on the pattern of gene expression and if this is related to reduced AF burden.
Statistical analysis
The primary endpoint is a reduction of AF burden in the intervention group compared to control. We have defined a 25% reduction of the AF burden as a clinical significant effect. The extent to which treatment of AF will improve SA is unclear, and there are few data on the effects of treating SA on arrhythmia burden in patients with AF. With a mean time of 34% in atrial fibrillation and a standard deviation (SD) of 12% [Citation29], a power of 80% and a two-sided significance level of 5% we will need at least 33 patients in each group. We will include a total of 100 patients in order to compensate for dropouts and to allow evaluation of secondary endpoints. No interim analysis is planned, unless there is a substantially increased number of serious adverse events in either of the study arms.
All analyses will be conducted on an intention-to-treat (ITT) basis. Comparison of means between the intervention and control group will be performed by Student`s t-test. Further, in secondary analyses, both parametric and non-parametric statistics will be used, depending on distribution of the data. Skewed variables will be analyzed by Kruskal Wallis, Friedmann, Mann-Whitney U or Wilcoxon Rank Sum and Spearman depending on design. Variables that are normally distributed will be analyzed by one-way and repeated measures ANOVA and paired and Student`s t-test. Contingency statistics will be performed as appropriate.
Discussion
PAF is associated with a high morbidity, of which stroke is the most feared complication. Current antiarrhythmic medications and ablation have limited efficacy, and identification of alternative triggers and treatments is warranted. SA is common in patients with PAF and the A3 study will investigate SA as a potential trigger for PAF. Effective treatment of SA has the potential to improve outcomes, as well as quality of life, in patients with paroxysmal AF and concomitant SA. However, the large event-driven randomized Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure (SERVE-HF) study unexpectedly demonstrated that ASV treatment of CSA in patients with systolic heart failure increased all-cause and cardiovascular mortality [Citation30]. The randomized Sleep Apnea Cardiovascular Endpoints (SAVE) study demonstrated that therapy with CPAP did not prevent CV events in patients with moderate-to-severe OSA and established CV disease [Citation31]. However, no such robust clinical trial data are yet available to show whether treatment of SA reduces the burden of AF. The effect of SA on triggering AF has recently been recognized but the impact is unclear and the impact of treatment has not been clearly defined. Thus, there is a need for a comprehensive prospective randomized study to determine whether treatment of SA reduces the burden of AF. The A3 study is (to our knowledge) the first prospective randomized study conducted in patients with paroxysmal AF and concomitant SA treated with PAP therapy.
The first patient was randomized in January 2016. As of November 1st 2018, 95 patients had been enrolled. Patient recruitment is expected to be complete by the end of 2018, with first results available in 2019.
Conclusions
The A3 study is a randomized trial that will assess for the first time whether treatment of SA will reduce the burden of AF. The study will also examine if SA treatment reduces the recurrence rate after ablation, which could lead to fewer re-ablations and reduce possible procedure complications for patients. An overall aim is better patient phenotyping, which could aid in a more person-specific treatment approach. The implanted recording device will provide objective data on which to base decisions about when to stop or start medication. The A3 trial should facilitate better understanding of the interactions between AF and SA. Potentially, our results might provide the rationale for new therapeutic strategies in patients with AF and might reduce the economic burden of AF.
Author contribution
All authors have conformed to the “Uniform Requirements for Manuscripts Submitted to Biomedical Journals”, prepared by the International Committee of Medical Journal Editors (ICMJE). All authors fulfil all criteria for being authors.
G.M.T., B.Ø., P.A., T.U., T.H.V., S.S., T.E., H.Z., S.A., H.A., O.G.A., J.P.L., L.G. contributed to the conception or design of the work. G.M.T., L.A., T.E.H., B.Ø., E.L., S.S., T.E., H.A., O.G.A., J.P.L., L.G. contributed to the acquisition, analysis, or interpretation of data for the work. G.M.T., L.G. drafted the manuscript. G.M.T., L.A., T.E.H., B.Ø., E.L., P.A., T.U., T.H.V., S.S., T.E., H.Z., S.A., H.A., O.G.A., J.P.L., L.G. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgements
We thank MSc PhD Ragnhild S. Falk of the Unit of Biostatistics and Epidemiology for statistical advice. English language editing assistance was provided by Nicola Ryan, independent medical writer, funded by ResMed. Members of the steering committee are listed in the Acknowledgements section.
Executive Steering Committee: Lars Gullestad, Ole-Gunnar Anfinsen, Harriet Akre, Jan Pål Loennechen, Sigurd Loe Steinshamn, Svend Aakhus
Disclosure statement
Dr Traaen has received speaker honoraria from ResMed Norway.
Additional information
Funding
References
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954.
- Malmo V, Nes BM, Amundsen BH, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473.
- Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060.
- Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231.
- Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689.
- Naughton MT. Sleep disorders in patients with congestive heart failure. Curr Opin Pulm Med. 2003;9:453–458.
- Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257.
- Imadojemu VA, Mawji Z, Kunselman A, et al. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413.
- Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia-implications for sleep apnea. Clin Exp Hypertens A. 1988;10: Suppl 1:413–422.
- Carpagnano GE, Kharitonov SA, Resta O, et al. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–1392.
- Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51.
- Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32:1009–1015.
- Carpagnano GE, Spanevello A, Sabato R, et al. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010;155:35–43.
- Steiropoulos P, Kotsianidis I, Nena E, et al. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 2009;32:537–543.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571.
- Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3:445–451.
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367.
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717.
- Hrubos-Strom H, Randby A, Namtvedt SK, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP). J Sleep Res. 2011;20:162–170.
- Cadby G, McArdle N, Briffa T, et al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest. 2015;148:945–952.
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300–305.
- Naruse Y, Tada H, Satoh M, et al. Radiofrequency catheter ablation of persistent atrial fibrillation decreases a sleep-disordered breathing parameter during a short follow-up period. Circ J. 2012;76:2096–2103.
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–337.
- Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012.
- Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3:141–147.
- Loge JH, Kaasa S, Hjermstad MJ, et al. Translation and performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. J Clin Epidemiol. 1998;51:1069–1076.
- Stavem K, Kjelsberg FN, Ruud EA. Reliability and validity of the Norwegian version of the Functional Outcomes of Sleep Questionnaire. Qual Life Res. 2004;13:541–549.
- Beiske KK, Kjelsberg FN, Ruud EA, et al. Reliability and validity of a Norwegian version of the Epworth sleepiness scale. Sleep Breath. 2009;13:65–72.
- Steven D, Rostock T, Lutomsky B, et al. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur Heart J. 2008;29:1037–1042.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931.
