Abstract
Objectives. We conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) including the comparison of non-vitamin K antagonist oral anticoagulants (NOACs) with warfarin for patients with atrial fibrillation (AF). Design. Network meta-analysis. Two authors independently extracted data. All authors evaluated overall confidence in the evidence. Results. Eighteen RCTs included in our review, a total of 78,796 patients with AF, with sample sizes from 90 to 21,105 patients. Apixaban 5 mg (OR: 0.79, 95% CI: 0.66 to 0.95), dabigatran 110 mg (0.91, 0.74–1.12), dabigatran 150 mg (0.66, 0.53–0.82), edoxaban 60 mg (0.87, 0.74–1.02), and rivaroxaban 20 mg (0.88, 0.74–1.03) reduced the risk of stroke or systemic embolism compared with warfarin. Dabigatran 150 mg had the highest P-score for reducing stroke or systemic embolic events. The risk of haemorhagic stroke and all-cause mortality was lower with all NOACs than with warfarin. Apixaban 5 mg (0.69, 0.60–0.80), dabigatran 110 mg (0.80, 0.69–0.93), dabigatran 150 mg (0.93, 0.80–1.08), edoxaban 30 mg (0.46, 0.40–0.54), and edoxaban 60 mg (0.78, 0.69–0.90) reduced the risk of major bleeding compared with warfarin. Edoxaban 30 mg had the highest P-score for reducing major bleeding. The plots of P-scores rank showed that apixaban offered the most favorable balance of efficacy and safety. Conclusions. This study adds an attempt for treatment ranking of both efficacy and safety outcomes. Future trials comparing directly NOACs are needed in order to provide conclusive proofs for these results and not only circumstantial evidence offered by a network meta-analysis.
Introduction
Over the last decade, several non-vitamin K antagonist oral anticoagulants (NOACs), which selectively inhibit thrombin or activated factor X have been developed for the anticoagulation treatment of patients with atrial fibrillation (AF). The 4 large phase III randomized controlled trials (RCTs) (RE-LY, ROCKET-AF, ARISTOTLE and ENGAGE AF-TIMI 48), as well as a large number of phase II RCTs and several meta-analyses have favored the use of NOACs over vitamin K antagonists in patients with non-valvular AF [Citation1–14].
Evidence-based guidance about the selection of NOACs for AF is not definitive. The comparative effectiveness of treatment regimens that include stepwise approaches is unclear, especially regarding high-risk patient groups, partially because of the scarcity of direct head-to-head trials. Furthermore, the effects of NOACs may have been overestimated as some patients treated with warfarin in clinical trials maintained without the therapeutic INR target of 2.0–3.0 [Citation6,Citation10]. For this purpose, a network meta-analysis (NMA) was performed in order to rank the NOACs and warfarin for each outcome.
Materials and methods
Data searches and study selection
This NMA was registered in PROSPERO with registration number: CRD42017078915 and was reported according to pertinent reporting guidelines for this type of study design [Citation15]. We searched for systematic reviews (SRs) examining the outcomes related to NOACs in AF treatment, when compared to warfarin.
A purposive literature search for SRs and RCTs took place from inception to September 3rd 2017. We screened MEDLINE (via PubMed), Cochrane Library and PROSPERO for SRs and clinicaltrials.gov in order to find further RCTs, only in English language. A more comprehensive search strategy was applied in MEDLINE, using medical subject headings (MeSH) (Supplementary material 1). For all included studies, reference lists were also examined. Two independent authors (CA and ID) screened the titles and abstracts against the eligibility criteria for inclusion.
We searched for SRs and meta-analyses, relevant to our topic and analyzed the full texts to extract the eligible RCTs. A third reviewer (ABH) intervened when there was a disagreement between the other two authors.
Data extraction and quality assessment
The extraction items from the two independent reviewers include: citation details; objectives of the included review; type of review; participant details; setting and context; number of databases sourced and searched; date range of database searching; number of studies, types of studies and country of origin of studies included in each review; instrument used to appraise the primary studies and the rating of their quality; outcomes reported that are relevant; method of synthesis/analysis employed to synthesize the evidence; major conclusions; metric used and effect size (for meta-analyses); confidence intervals (for meta-analyses). Authors were contacted in case of missing data. If the requested data could not be retrieved, the study was not included in the analysis. Data from the individual RCTs were extracted from the included SRs for the NMA.
The risk of bias of the retrieved RCTs, was assessed with the Cochrane risk of bias tool [Citation16]. Six risk of bias domains were assessed, including sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessments, incomplete outcome data, and selective outcome reporting.
Statistical methods (network Meta-analysis)
NMA was conducted to assess the efficacy and tolerability in two primary (stroke or systemic embolism, major bleeding) and six secondary outcomes (clinically relevant non-major bleeding, gastrointestinal bleeding, myocardial infarction, haemorhagic stroke, ischaemic stroke, all-cause mortality). Direct evidence from two-armed and three-armed RCTs were used to construct treatment networks for each outcome and indirect effect measures were calculated to incorporate the pooled effect of each treatment node. Random-effect NMA was performed based on a graph theoretical approach within a frequentist framework [Citation17]. In the absence of direct comparisons in RCTs, another method to investigate the comparative effect of the interventions of interest is to perform indirect comparisons using a common comparator, warfarin in our case. NMA was performed with R version 3.3.3. using package “netmeta”. Ranking interventions was determined by using P-scores. For each outcome, P-scores allow ranking the treatments on a continuous 0–1 scale and so, it was determined which treatment was the best, the second best, etc [Citation18]. Homogeneity and consistency were assessed by decomposing the Q statistic into variation of the effect estimates within designs (heterogeneity) and between designs (inconsistency). Inconsistency between direct and indirect evidence was investigated by “node-splitting” [Citation19].
Results
Search results
We found eighteen RCTs [Citation1–10,Citation20–27] included a total of 78,796 patients with AF, with sample sizes varying from 90 to 21,105 (). None of them was head-to-head trial. presents PICOS elements of the individual RCTs. shows the patterns of comparison between the different treatments for two primary outcomes (stroke or systemic embolic events and major bleeding). Both networks have similar geometry, with warfarin acting as the common comparator. Among all the included trials, only 4 (22%) were double blinded, and only 2 (11%) had reported no funding from the industry (Supplementary material 2).
Figure 2. (A) Network plot for stroke or systemic embolic events. (B) Network plot for major bleeding. The size of nodes is proportional to the number of studies.
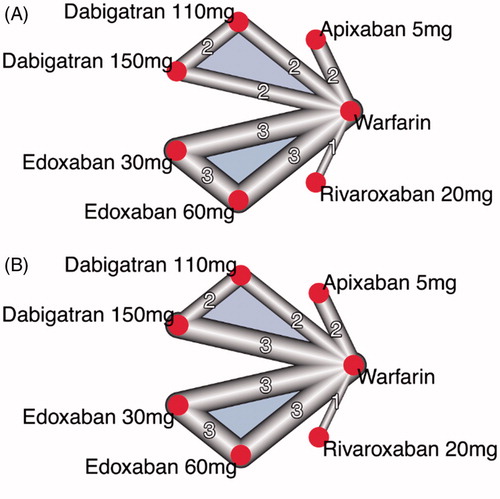
Table 1. PICOS Elements of the individual RCTs.
Network Meta-analysis
Warfarin was used as common comparator since it was the most common treatment among trials. Comparisons between NOACs and warfarin on the risk of stroke or systemic embolism and major bleeding are shown in . Dabigatran at 150mg was found to have the highest P-score for stroke or systemic embolism (followed by apixaban 5mg, edoxaban 60mg, rivaroxaban 20mg, dabigatran 110mg, warfarin and edoxaban 30mg). Different results were observed as regards the risk of major bleeding: Edoxaban, dosed at 30mg, had the highest P-score for major bleeding events. The remaining treatments were ranked as follows: apixaban 5mg, edoxaban 60mg, dabigatran 110mg, dabigatran 150mg, warfarin and rivaroxaban 20mg. Heterogeneity and inconsistency for all outcomes was zero. Odds ratios as calculated from the fitted models for the two primary outcomes are summarized in .
Figure 3. Comparisons between non-vitamin K antagonists and warfarin on the (A) stroke or systemic embolic events, and (B) major bleeding.
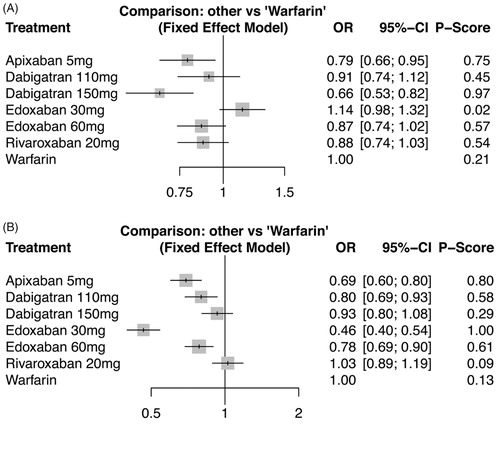
Table 2. Odds ratios with 95% confidence intervals as calculated from the fitted models for major bleeding and stroke or systemic embolism.
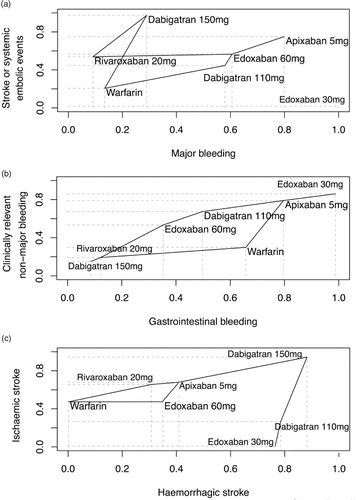
All the safety events showed that edoxaban 30mg was the treatment causing less bleeding episodes. Moreover, rankings for stroke events demonstrated that haemorrhagic and ischemic stroke were less frequent under dabigatran 150mg (Supplementary material 3 for all secondary outcomes). The plots of P-scores rank () show that apixaban offered a favourable balance of efficacy and safety. As regards the myocardial infarction, dabigatran at the dose of 150mg was related with a significantly increased risk, while dabigatran 110mg was close to statistical significance. On the other hand, concerning the risk of haemorhagic stroke, all NOACs were better than warfarin.
Discussion
Our NMA of RCTs shows that NOACs appear to be at least as effective as warfarin in reducing the risk of stroke in patients with atrial fibrillation. Apixaban 5 mg and edoxaban 60 mg reduced more the risk of bleeding episodes compared with warfarin, while maintain efficient in reducing the risk of stroke. Although the RCTs have included patient populations with different clinical characteristics and this should not be interpreted as a head-to-head comparison between the NOACs, this work adds an attempt for treatment ranking for both efficacy and safety outcomes to the current literature.
The inclusion of a great number of RCTs does not affect the heterogeneity and the inconsistency, as they were equal to zero for all outcomes. Contrary to other recent meta-analyses [Citation28], our aim was not to include RCTs comparing drugs that are not indicated for the antithrombotic treatment of AF (antiplatelets) [Citation10]. The inclusion of these RCTs in a NMA would inaccurately affect its results. To our knowledge, our NMA has the biggest number of included primary studies compared to the already existing NMA, which had included only the above category of RCTs.
Our findings about the overall efficacy and safety of NOACs are consistent with previously published meta-analyses [Citation29–31]. As regards dabigatran, trials show that different cut-off values of achieved drug levels may play a role in the efficacy and safety of this drug. [Citation32]. This may reduce the convenience of this νOAC and increase its cost, but it can be used for individualization of the dosage in certain cases. Furthermore, there have been doubts about the efficacy data for rivaroxaban from ROCKET-AF [Citation33]. However, the FDA concluded that the effects on stroke or bleeding rates were minimal. The above evidences may not be aggravating for these NOACs. However, the same evidences increase further the need for trials, which will compare NOACs directly, and conclude to which NOAC should be used at each patients’ subgroup in the everyday clinical practice. Consequently, conclusive proofs will be provided and not only circumstantial evidence offered by a network meta-analysis.
Study limitations
The main limitations of this NMA is the number of studies and the complete lack of direct comparisons among the NOACs. All treatments are compared to warfarin or their respective substance at a lower dose. This deprives the constructed networks from crucial information needed to infer on the consistency of the network and the reliability of the results. The current NMA was conducted despite the violation of the transitivity assumption. The fact that all RCTs were funded by industry certainly cause more favorable results due to reporting bias. No meta-regression analysis was attempted to investigate the effect of patient characteristics in individual studies on the pooled outcome.
Conclusions
This study adds an attempt for treatment ranking for both efficacy and safety outcomes. However, no comparative conclusions should be drawn from this NMA. Future trials directly comparing NOACs are needed to infer in efficacy and investigate potential effect modifiers that could be used for patient-targeted therapy.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgments
CA: Proposed the structure of the paper, critically appraised the paper and made the final suggestions
ID: Proposed the idea, proposed the statistical analysis and formulate the paper
EA: Proposed the statistical analysis
FIE: Critically appraised the paper, made the final suggestions
PV: Critically appraised the paper, made the final suggestions
ABH: Supervised the statistical analysis, critically appraised the paper and made the final suggestions
VK: Critically appraised the paper, made the final suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007;100:1419–1426.
- Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J. 2012;76:2104–2111.
- Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. -The ARISTOTLE-J study. Circ J. 2011;75:1852–1859.
- Weitz JI, Connolly SJ, Patel I, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104(3):633–41.
- Chung N, Jeon HK, Lien LM, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost. 2011;105(3):535–44.
- Yamashita T, Koretsune Y, Yasaka M, et al. Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J. 2012;76:1840–1847.
- Caldeira D, Barra M, Ferreira A, et al. Systematic review with meta-analysis: the risk of major gastrointestinal bleeding with non-vitamin K antagonist oral anticoagulants. Aliment Pharmacol Ther. 2015;42:1239–1249.
- Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 2012;126:2381–2391.
- Jia B, Lynn HS, Rong F, et al. Meta-analysis of efficacy and safety of the new anticoagulants versus warfarin in patients with atrial fibrillation. J Cardiovasc Pharmacol. 2014;64:368–374.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784.
- Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324.
- Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58.
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Statist Med. 2010;29:932–944.
- Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–1811.
- Cappato R, Ezekowitz MD, Klein AL, et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355.
- Calkins H, Willems S, Gerstenfeld EP, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636.
- Kuwahara T, Abe M, Yamaki M, et al. Apixaban versus warfarin for the prevention of periprocedural cerebral thromboembolism in atrial fibrillation ablation: multicenter prospective randomized study. J Cardiovasc Electrophysiol. 2016;27:549–554.
- Nin T, Sairaku A, Yoshida Y, et al. A randomized controlled trial of dabigatran versus warfarin for periablation anticoagulation in patients undergoing ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2013;36:172–179.
- NCT01136408. A dose response study of dabigatran etexilate (BIBR 1048) in pharmacodynamics and safety in patients with non-valvular atrial fibrillation in comparison to warfarin. Clinicaltrialsgov. https://clinicaltrials.gov/ct2/show/NCT01136408.
- NCT00973245. BAY59-7939 in Atrial Fibrillation Once Daily (OD). Clinicaltrialsgov. https://clinicaltrials.gov/ct2/show/NCT00973245.
- NCT00973323. BAY59-7939 Japanese in Atrial Fibrillation (2nd). Clinicaltrialsgov. https://clinicaltrials.gov/ct2/show/NCT00973323.
- Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058.
- Rasmussen LH, Larsen TB, Graungaard T, et al. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097.
- Dogliotti A, Paolasso E, Giugliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart. 2014;100:396–405.
- Lip GY, Mitchell SA, Liu X, et al. Relative efficacy and safety of non-Vitamin K oral anticoagulants for non-valvular atrial fibrillation: Network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94.
- Huisman MV, Lip GY, Diener HC, et al. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–847.
- Cohen D. Manufacturer failed to disclose faulty device in rivaroxaban trial. BMJ. 2016;354:i5131.