Abstract
Objectives. The study sought to assess the prognostic impact of recurrences of electrical storm (ES-R) on mortality, rehospitalization and major adverse cardiac events (MACE). Background. Data on the prognostic impact of ES-R is rare. Methods. All consecutive ES patients with an implantable cardioverter defibrillator (ICD) were included retrospectively from 2002 to 2016. Patients with ES-R were compared to patients without ES-R. The primary endpoint was all-cause mortality, secondary endpoints were in-hospital mortality, rehospitalization and MACE. Results. A total of 87 consecutive ES patients with an ICD were included, of which 26% presented with ES-R at 2.5 years of follow-up. ES-R patients revealed lower LVEF compared to non-ES-R patients (91% vs. 61%; p = .081). There was a numerically higher rate of the primary endpoint of all-cause mortality at 2.5 years (50% vs. 32%; log-rank p = .137). Furthermore, ES-R was associated with increasing rates of rehospitalization (64% vs. 37%; p = .031; HR 1.985; 95% CI 1.025–3.845; log-rank p = .042), especially of acute heart failure (32% vs. 12%; p = .001; HR 3.262; 95% CI 1.180–9.023; log rank p = .023). MACE were higher in ES-R patients (55% vs. 35%; p = .113; log rank p = .141). ES patients with LVEF ≤35% were 12.4 times more likely to develop ES-R (HR 12.417; 95% CI 1.329–115.997; p = .027). Conclusion. At long-term follow-up of 2.5 years, ES-R was associated with numerically higher rates of long-term all-cause mortality and significantly higher rates of rehospitalization due to acute heart failure. LVEF ≤35% was associated with increased risk of ES-R.
Condensed Abstract
This study examined retrospectively the impact of recurrences of electrical storm (ES-R) on survival in 87 patients. ES-R was associated with numerically higher long-term all-cause mortality, whereas significantly higher rates of rehospitalization, respectively of acute heart failure were observed.
ES-R is associated with numerically higher rates of all-cause mortality at long-term follow-up.
ES-R is associated with significantly higher rates of rehospitalization and numerically higher rates of MACE at long-term follow-up, mainly due to acute heart failure.
Patients with LVEF ≤35% were 12.4 times more likely to develop ES-R.
Highlights
Introduction
Ventricular tachyarrhythmias are serious and life-threatening heart rhythm disorders. The implantation of internal cardioverter defibrillators (ICD) has demonstrated prognostic benefit compared to sole antiarrhythmic pharmacotherapy for secondary prevention of sudden cardiac death (SCD) after life-threatening sustained ventricular arrhythmias [Citation1]. Electrical storm (ES) is defined as ≥3 distinct episodes of sustained ventricular tachycardia (VT) or fibrillation (VF) requiring ICD therapy within 24 hours [Citation2]. In ICD recipients ES is present in up to 20% [Citation3], with a lower prevalence of 4% in primary preventive and up to 28% in secondary preventive ICDs [Citation4].
Clinical presentation of patients with ES may differ from asymptomatic delivery of anti-tachycardia pacing to hemodynamic instability with multiple ICD-related shocks. Presence of ES was recently shown to be associated with an increased risk of hospitalization and death compared to patients with a history of ventricular tachyarrhythmia [Citation5]. Antiarrhythmic drugs, such as amiodarone, azimilide or dofetilide, overdrive ventricular pacing, hemodynamic support and sympathetic blockade by either beta-blocker or interventional sympathetic denervation represent established first-line therapies in ES patients. Furthermore, percutaneous catheter ablation has been recommended as another causative treatment option in selected patients [Citation6].
Until now, the exact pathophysiology and characteristics of patients developing ES are yet not fully understood [Citation7]. However, several studies have made attempts to identify potential triggers and risk factors for ES development, whereas their causative role has not been explained [Citation8]. Impaired left ventricular ejection fraction (LVEF) ≤30%, lack of angiotensin converting enzyme (ACE) inhibitor therapy and age above 65 years may be independent predictors for recurrences of electrical storm (ES-R) [Citation9]. However, further data about patients with ES-R and potential related risk factors are warranted.
Therefore, the present study sought to investigate retrospectively the impact of ES-R on long-term outcomes including mortality within a single-centre cohort of consecutive ES patients.
Methods
Study population
The present study included all consecutive patients presenting with ES from 2002 until 2016 at one institution. Electrical storm was defined as ≥3 episodes of ventricular tachyarrhythmias delimited by at least 5 minutes leading to appropriate ICD therapy within 24 hours [Citation2]. ES-R was defined as the recurrence of further episodes of ES at follow-up beyond the initial 24 hours of prior ES. Multiple appropriate ICD therapies to terminate one single episode of VT/VF have been considered as part of one arrhythmic episode. All ICDs were allowed, transvenous, subcutaneous (s-ICD) and cardiac resynchronization therapy with defibrillator function (CRT-D).
Ventricular tachyarrhythmias comprised VT and VF, as defined by current international guidelines [Citation10]. Sustained VT was defined by duration of more than 30 seconds or causing hemodynamic collapse within 30 seconds, non-sustained VT by duration of less than 30 seconds both with wide QRS complexes (≥120 milliseconds) at a rate greater than 100 beats per minute [Citation10]. Ventricular tachyarrhythmias were documented by 12-lead ECG, ECG tele-monitoring, ICD or in case of unstable course or during resuscitation by external defibrillator monitoring. Documented VF was treated by external defibrillation and in case of prolonged instability with additional intravenous anti-arrhythmic drugs during cardiopulmonary resuscitation (CPR).
All relevant clinical data were documented using the electronic hospital information system, ICD protocols, discharge letters, daily charts, patients´ files and reports from diagnostic testing. In detail, data documentation comprised baseline characteristics, prior medical history, prior medical treatment, length of index stay, detailed findings of laboratory values at baseline, data derived from all non-invasive or invasive cardiac diagnostics and device therapies, such as coronary angiography, electrophysiological examination, as well as imaging modalities, such as echocardiography or cardiac magnetic resonance imaging (cMRI). Documentation period lasted from index event until 2016. Documentation of all medical data was performed by independent cardiologists at the time of the patients´ individual period of clinical presentation, being blinded to final data analyses.
The present study is derived from an analysis of the “Registry of Malignant Arrhythmias and Sudden Cardiac Death - Influence of Diagnostics and Interventions (RACE-IT)” and represents a single-center registry including consecutive patients presenting with ventricular tachyarrhythmias and sudden cardiac arrest being admitted acutely to the University Medical Center Mannheim (UMM), Germany (clinicaltrials.gov identifier: NCT02982473) from 2002 until 2016. The registry was carried out according to the principles of the declaration of Helsinki and was approved by the medical ethics committee II of the Faculty of Medicine Mannheim, University of Heidelberg, Germany.
Definition of endpoints
The primary endpoint was all-cause mortality during the follow-up period until 2016. All-cause mortality was documented using our electronic hospital information system and by directly contacting state resident registration offices (“bureau of mortality statistics”) across Germany. Identification of patients was verified by place of name, surname, day of birth and registered living address.
Secondary endpoints comprised in-hospital mortality, first rehospitalization and major adverse cardiac events (MACE). First rehospitalization comprised first rehospitalization due to VT, VF, CPR, acute heart failure or acute myocardial infarction (AMI). MACE were defined as the composite of AMI, target vessel revascularization by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) and all-cause mortality [Citation11].
Statistical methods
Quantitative data are presented as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data and were compared using the Student’s t test for normally distributed data or the Mann-Whitney U test for nonparametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov-Smirnov test. Spearman’s rank correlation for nonparametric data was used to test univariate correlations. Qualitative data are presented as absolute and relative frequencies and compared using the Chi2 test or the Fisher’s exact test, as appropriate.
The following analyses were applied stepwise to evaluate the prognostic value of predefined variables for all-cause mortality: Kaplan-Meier survival curves were calculated with log-rank testing for statistical significance. Uni-variable hazard ratios (HR) are given together with 95% confidence intervals. Multivariable Cox regression models with ES-R as the dependent variable were developed using the “forward selection” option, where only statistically significant variables (p < .05) were included and analyzed simultaneously.
The result of a statistical test was considered significant for p < .05. SAS, release 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistics.
Results
Study population
A total of 87 consecutive patients with at least one episode of ES were included. 22 of 87 ES patients developed ES-R at follow-up (26%) with an average time of 5.3 months between ES and ES-R.
outlines the baseline characteristics. Slightly numerically higher rates of coronary artery disease (69% vs. 59%), cardiogenic shock (3% vs. 0%) and CPR (8% vs. 0%) were seen in ES patients. In contrast, significantly higher rates of cardiomyopathy (46% vs. 20%; p = .019) respectively dilated cardiomyopathy (46% vs. 14%; p = .019), as well as infectious causes (18% vs. 2%; p = .014) were seen in ES-R patients. Furthermore, numerically higher numbers of long QT syndrome were seen in ES-R patients (5% vs. 0%), whereas Brugada syndrome was seen more often in ES patients (3% vs. 0%).
Table 1. Baseline characteristics.
Non-ES-R patients were tested more often by electrophysiological examinations (29% vs. 14%) and accordingly VT ablation was performed more often in non-ES-R patients (23% vs. 14%) ().
Both ES-R and non-ES-R patients were discharged with equal rates of betablockers (95%) and ACE inhibitors/AT1-receptor antagonist (ARB) (79% vs. 81%). In contrast, ES-R patients were more often treated with amiodarone compared to non-ES-R patients (71% vs. 48%, p = .058). ES-R patients revealed numerically higher PQ, QT and QRS intervals. Accordingly, ES-R patients had lower LVEF (LVEF ≥55%: 14% vs. 5%; LVEF 45–54%: 10% vs. 5%; LVEF 35–44%: 15% vs. 0%; LVEF ≤35%: 61% vs. 91%; p = .081). Most patients had ICD implantation for secondary prevention, especially in ES-R patients (73% vs. 58%). Conventional transvenous ICD was the most common type of ICD (87%), followed by CRT-D and sICD (range 3–9%) ().
Primary endpoint – all-cause mortality
Follow-up regarding the primary endpoint of all-cause mortality at 2.5 years was completed for all patients (median 2.45 years (IQR 1.01–4.77 years)). ES-R patients were associated with a numerically higher rate of the primary endpoint compared to non-ES-R patients (all-cause mortality at 2.5 years: 50% vs. 32%, p = .137; log-rank p = .301; ; , left panel). Also after adjustment for patients who did not survive first ES, mortality rates still remain not statistically significant.
Figure 1. Prognostic impact of ES-R on all-cause mortality (left panel), overall rehospitalization (middle panel) and MACE (right panel) at long-term follow-up.
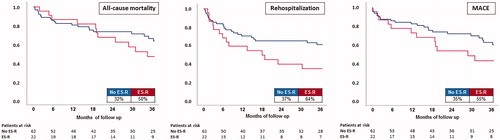
Table 2. Primary and secondary endpoints.
Secondary endpoints
No difference was observed regarding in-hospital mortality between ES-R and non-ES-R patients (3% vs. 0%, p = 1.000; ).
In contrast, at 2.5 years of follow-up ES-R patients revealed significantly higher rates of overall first rehospitalization (64% vs. 37%, p = .031), which was mainly due to acute heart failure (32% vs. 12%; p = .001; HR 3.262; 95% CI 1.180–9.023; log rank p = .023). Notably, in about a quarter of all patients with ES-R first rehospitalization was related to ES-R (; , middle panel). ES-R patients were 2 times more likely to be rehospitalized (HR 1.985; 95% CI 1.025–3.845; p = .042).
Finally, rates of MACE were numerically higher in ES-R patients during follow-up, however did not reach statistical significance (55% vs. 35%; p = .113; log-rank p = .141) (; , right panel).
Multivariable Cox regression
Multivariable Cox regression revealed LVEF ≤35% as the most robust prognostic risk factor being associated with ES-R in the present study cohort. Patients with LVEF ≤35% were 12.4 times more likely to develop ES-R (HR 12.417; 95% CI 1.329–115.997; p = .027). Age, male sex, diabetes, impaired renal function (defined as creatinine >106 µmol/L) and coronary artery disease were not associated with ES-R in this population-based model. Notably, in multivariable Cox regression VT ablation did not affect rates of ES-R ().
Table 3. Multivariable Cox regression model to evaluate prognostic factors influencing ES-R with LVEF as discrete variable.
Even in Cox regression models with LVEF as a continuous variable, LVEF remained the only significant variable associated with ES-R (HR 3.390; 95% CI 1.022–11.237; p = .046; ).
Table 4. Multivariable Cox regression model to evaluate prognostic factors influencing ES-R with LVEF as continuous variable.
Discussion
The present study investigates the prognostic impact of ES-R on all-cause mortality, rehospitalization rates and MACE in consecutive ES patients at long-term follow-up. It was demonstrated that ES-R patients were associated with numerically higher rates of all-cause mortality at long-term follow-up compared to non-ES-R patients. Furthermore, ES-R patients had higher rates of rehospitalization, mainly due to acute heart failure. Numerically higher rates of MACE were observed in ES-R patients. Accordingly, ES patients with LVEF ≤35% were associated with highest risk of future ES-R even after multivariable adjustment, where LVEF ≤35% remained the only significant prognosticator for ES-R.
To the authors' best knowledge, the prognostic impact of ES-R compared to non-ES-R has never been investigated before. Previously published studies analysed the incidence and prognostic implication of ES in ICD recipients and referred to inconsistent definitions of ES and ES-R [Citation3,Citation12,Citation13]. Despite the investigation of exact frequencies and time ranges in between ES episodes, applied ES definitions were adapted to ≥2 episodes of VT/VF within 24 hours [Citation12,Citation13] instead of the generally accepted definition of ES including ≥3 episodes of VT/VF within 24 hours [Citation2]. Percutaneous catheter ablation of ES was demonstrated repeatedly to improve survival rates. A meta-analysis about catheter ablation of VT including 3 randomized controlled trials with a total of 346 patients demonstrated a reduced rate of consecutive ICD-related shocks and ES-R, whereas no decrease of all-cause mortality or MACE was proven at 2.1 years [Citation14]. A recent large multicentre study found ablation after ES to be associated with a reduction of recurrences and improved one-year survival [Citation15]. This is in contrast to the results of the present study, in which ES-R rates were not affected by VT ablation. However, in our study sample size was small and VT ablation rates were low (only 3 patients with ES-R were ablated). Notably, all 3 patients with VT ablation were free from VT recurrences within the follow-up period.
The association of ES with increasing mortality was demonstrated repeatedly in various studies including preselected patients with idiopathic dilative cardiomyopathy or patients after myocardial infarction [Citation15,Citation16]. Especially in patients with a structural heart disease the presence of ES was associated with higher mortality compared to non-ES patients [Citation16]. Patients undergoing left ventricular assist device implantation (LVAD), e.g. with the HeartMate II device, were associated with increasing rates of ES and associated 30-days all-cause mortality. However, evaluation of ES-R was beyond the scope of the latter study [Citation17]. Data from the MADIT II trial demonstrated an increasing mortality risk in ES patients, where ES revealed the most important independent risk factor of mid-term cardiac death verified in 27 ES patients [Citation15]. In line with the data of the present study, ES itself might reveal stronger prognostic impact compared to non-ES patients (as previously published by other working groups). The present study only found numerically higher rates of all-cause mortality at long-term follow-up, which may be related to the smaller sample size of this single centre cohort.
The clinical presentation of ES differs dramatically. In about 80% hospitalization is required in ES patients. In case of shock delivery of more than three times within 24 hours the rate of rehospitalization was reported to increase up to 100% [Citation18]. Within the present study the increasing overall rehospitalization rate was mainly attributed to acute heart failure in ES-R patients. This is consistent with previous observations of Guerra et al. suggesting ES as a clinical manifestation of worsening acute heart failure [Citation19]. The present study confirms these findings, since ES-R patients revealed a numerically lower baseline LVEF compared non-ES-R patients, and LVEF ≤35% itself was the only risk factor being associated with ES-R at long-term follow-up.
Low LVEF represents a major risk factor for increased mortality and hospitalization rates in post myocardial infarction patients [Citation20]. Specifically regarding at ES studies demonstrated significant associations with low LVEF [Citation12], which was not consistently proven in another study by Hohnloser et al. [Citation21]. Usually, these studies are of smaller patient size (estimated average range: 15–150 ES patients being included) and focus on ischaemic cardiomyopathy as the most prevalent disease entity. A recent study from our own institution found heart failure (LVEF <35%) not to be predictive for first ES, but for more than one future ES-R event [Citation9]. However, the results were derived from an overall ICD population with and without ventricular tachyarrhythmias, whereas the present study focuses on all consecutive ES occurring in between 2002 and 2016 at our institution. Accordingly, the results of the present study deliver novel insights and enforce the hypothesis that LVEF ≤35% might contribute to the clinical presentation of ES-R accompanied by acute heart failure in many cases within a prespecified cohort of consecutive ES patients only.
CAD was more present in patients without ES-R, whereas patients with ES-R suffered more often from cardiomyopathies. Percutaneous coronary interventions (PCI) of atherosclerotic lesions might eliminate ischemic triggers for ES. Cardiomyopathies and other non-ischemic triggers for ES might be treated insufficiently resulting in higher rates for ES-R.
The prognostic impact of ES-R on all-cause mortality, rehospitalization rates and MACE compared to non-ES-R at long-term follow up has never been investigated before. Therefore, this study provides novel insights on the prognostic impact of ES-R. Although ES itself might decrease survival rates significantly, ES-R may not impact mortality to the same extend.
Study limitations
The present study is based on a retrospective and observational single-centre registry. Rehospitalization rates were only documented within our own institution. Results may not be over-interpreted because sample size of ES-R patients is small. This is due to the naturally low prevalence of ES-R. Small sample size may have led to lower statistical power. Increase in sample size might lead to more statistically significant differences, even with regard to the primary endpoint of long-term all-cause mortality. Ablation rates among ES patients were low. This might be due to the heterogeneity of possible ES triggers (hypokaliaemia, hypoxia, cardiomyopathies) in our study. Low VT ablation rates might prevent to indicate beneficial outcome in ablated ES patients. Future multi-centre registries or even prospective randomized controlled trials are needed to clarify further the prognostic impact of ES-R.
Conclusions
ES-R was associated with numerically higher all-cause mortality and MACE, as well as with significantly higher rates of rehospitalization, especially due to acute heart failure. LVEF ≤35% was associated with increasing rates of ES-R.
Disclosure statement
The authors declare that they do not have any conflict of interest.
References
- Kuck KH, Cappato R, Siebels J, et al. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748–754.
- Israel CW, Manegold JC. [Electrical storm: definition, prevalence, causes and prognostic implications]. [Article in German]. Herzschr Elektrophys. 2014;25:59–65.
- Villacastin J, Almendral J, Arenal A, et al. Incidence and clinical significance of multiple consecutive, appropriate, high-energy discharges in patients with implanted cardioverter-defibrillators. Circulation 1996;93:753–762.
- Credner SC, Klingenheben T, Mauss O, et al. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998;32:1909–1915.
- Guerra F, Shkoza M, Scappini L, et al. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014;16:347–353.
- Rivard L, Andrade J. Innovative approaches to arrhythmic storm: the growing role of interventional procedures. Can J Cardiol. 2017;33:44–50.
- Tsuji Y, Heijman J, Nattel S, et al. Electrical storm: recent pathophysiological insights and therapeutic consequences. Basic Res Cardiol. 2013;108:336
- Huang DT, Traub D. Recurrent ventricular arrhythmia storms in the age of implantable cardioverter defibrillator therapy: a comprehensive review. Prog Cardiovasc Dis. 2008;51:229–236.
- Streitner F, Kuschyk J, Veltmann C, et al. Predictors of electrical storm recurrences in patients with implantable cardioverter-defibrillators. Europace 2011;13:668–674.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867.
- Kip KE, Hollabaugh K, Marroquin OC, et al. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:701–707.
- Brigadeau F, Kouakam C, Klug D, et al. Clinical predictors and prognostic significance of electrical storm in patients with implantable cardioverter defibrillators. Eur Heart J. 2006;27:700–707.
- Verma A, Kilicaslan F, Marrouche NF, et al. Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electr. 2004;15:1265–1270.
- Atti V, Vuddanda V, Turagam MK, et al. Prophylactic catheter ablation of ventricular tachycardia in ischemic cardiomyopathy: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electr. 2018;53:207–215.
- Sesselberg HW, Moss AJ, McNitt S, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm. 2007;4:1395–1402.
- Noda T, Kurita T, Nitta T, et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol. 2018;255:85–91.
- Corre J, Picard F, Garcia R, et al. Electrical storm in the early phase of HeartMate® II device implantation: incidence, risk factors and prognosis. Arch Cardiovasc Dis. 2018;111:332–339.
- Bansch D, Bocker D, Brunn J, et al. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol. 2000;36:566–573.
- Guerra F, Flori M, Bonelli P, et al. Electrical storm and heart failure worsening in implantable cardiac defibrillator patients. Europace 2015;17:247–254.
- Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–1433.
- Hohnloser SH, Al-Khalidi HR, Pratt CM, et al. Electrical storm in patients with an implantable defibrillator: incidence, features, and preventive therapy: insights from a randomized trial. Eur Heart J. 2006;27:3027–3032.
