Abstract
Objective. Limited data are available regarding prognostic value of nitric oxide metabolites (NOx) for clinical hard end points. In this study, we defined optimum cut-off values of serum NOx for predicting all-cause and cardiovascular disease (CVD) mortality events and prospectively investigated their hazards in the presence of traditional risk factors. Design. Serum NOx concentrations were measured at baseline (2006–2008) and 3520 adult men and women were followed during 7.7 years for all-cause and cardiovascular disease (CVD) mortality. To determine the optimal cut-off points of serum NOx, the receiver operator characteristic (ROC) curve analysis was used. Multivariate Cox proportional hazard models were used to estimate the hazard ratios (HRs) with 95% confidence intervals (95% CIs) of all-cause and CVD mortality below and above the defined optimal cut-off points of serum NOx. Results. Mean age of participants was 44.5 ± 16.0 years at baseline and 40.2% were male. Median (inter-quartile range) of serum NOx levels was 25.0 µmol/L (19.0–37.0), at baseline. The optimal cut-off points of serum NOx levels for predicting CVD and all-cause mortality were 30.5 and 32.5 µmol/L, respectively. In the presence of age, sex, body mass index, smoking, type 2 diabetes, hypertension, and history of CVD, a significant increased risk of CVD mortality (HR = 1.98, 95% CI = 1.10–3.58) and all-cause mortality (HR = 1.52, 95% CI = 1.05–2.21) was observed for serum NOx values higher than their cut-offs. Conclusion. Serum NOx level may be predictor of CVD mortality and death, in general populations.
Introduction
Cardiovascular diseases (CVD) are the leading cause of morbidity and mortality worldwide and was responsible for 31% of all global deaths in 2015 [Citation1]. By 2030, it has been predicted that over 23.3 million CVD-death will annually occur, and of which, over 80% will take place in developing countries [Citation2]. Accordingly, improved strategies for the primary prevention of CVD and identifying biomarkers and predictors of CVD burden are public health priorities [Citation3].
Up to 20% of CVD events are not caused by traditional risk factors (e.g. diabetes, dyslipidemia, hypertension, smoking), and these criteria could not appropriately categorize subjects for risk burden on the epidemiologic level [Citation4,Citation5]. Determining novel risk markers for predicting CVD events and non-communicable disease (NCD)-mortality has therefore significant potential to improve the selection of individuals for preventative strategies [Citation3].
Nitric oxide (NO) metabolism is suggested to be linked with several physiological and pathophysiological pathways [Citation6–8]. Interrupted NO pathways, including either reduced or elevated production of NO, have been reported as a risk factor and/or prognosis for development of chronic disorders especially CVD, renal dysfunction, diabetes, hypertension (HTN), and different types of cancer [Citation9–14]. Excessive NO production is mainly attributed to induction of inducible NO synthase (iNOS) during oxidative stress and inflammatory conditions, the situations that play crucial roles in development of pathological conditions [Citation15].
An elevated NO production induced by iNOS expression has been shown to contribute to increased rate of mortality [Citation16]. Some limited human evidence also imply that elevated serum NO metabolites (NOx), can predict the risk of CVD mortality [Citation17]. High basal NOx in combination with higher serum interlukine-6, was also associated with all-cause and CVD death in hemodialysis patients [Citation18].
Considering the increasing reports regarding predictive value of circulating NO for different chronic diseases, determining of its population-specific cut-off values for predicting health outcomes seems to be essential. The reference values for serum NOx concentration in our population was previously defined as 11.5–76.4 and 10.1–65.6 μmol/L in men and women, respectively; values above the upper limits have been shown to predict type 2 diabetes and metabolic syndrome [Citation19]. Increased serum NOx levels could also predict the occurrence of hypertriglyceridemic waist phenotype, chronic kidney disease and CVD events in our population, in a short-term follow-up [Citation20–22].
In this study, we aimed to define optimum cut-off values of serum NOx for predicting CVD and all-cause mortality events and prospectively investigated their hazards in a large community-based population.
Material and methods
Study population
This study was conducted within the framework of the Tehran Lipid and Glucose Study (TLGS). Briefly, TLGS is an ongoing community-based prospective study aiming to investigate and prevent non-communicable diseases, in a representative sample in the district 13 of Tehran, the capital city of Iran [Citation23]. From 10224 participants aged ≥19 y at baseline examination (2006–2008), 3541 participants, with complete data on serum NOx levels were included in the analysis. Participants who had no follow-up data (n = 21) were excluded and finally 3520 participants (1414 men and 2106 women) remained to be followed for all-cause and CVD mortality outcomes till March 2014 (median follow-up of 7.68 years, interquartile range = 7.32–8.04).
Demographic and anthropometric measures
Demographic data, smoking habits, and medical history (history of disease and use of medications for hypertension, type 2 diabetes and CVD) were collected by the trained interviewers. Details of anthropometric measurements including weight, height and waist circumference (WC), systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements have been described elsewhere [Citation24]. Assessment of physical activity levels was done using the Modifiable Activity Questionnaire (MAQ); the frequency and time spent on light, moderate, hard and very hard intensity activities according to the list of common activities of daily life over the past year were documented. Reliability and convergent validity of the Persian version of the MAQ has previously been investigated [Citation25]. Physical activity levels were expressed as metabolic equivalent hours per week (MET-h/week).
Biochemical measures
To measure biochemical variables, fasting blood samples were taken after 12–14 h, from all study participants at baseline and follow-up examinations. Fasting serum glucose (FSG) was measured by the enzymatic colorimetric method using glucose oxidase. The standard 2 hours serum glucose (2–h SG) test, using 75 g oral glucose load, was performed for all individuals who were not on anti-diabetic drugs. Serum triglyceride (TG) levels were measured by enzymatic colorimetric analysis with glycerol phosphate oxidase. High-density lipoprotein cholesterol (HDL-C) was measured after precipitation of the apolipoprotein B containing lipoproteins with phosphotungstic acid. Serum creatinine levels were assayed using kinetic colorimetric Jaffe method. Analyses were performed using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, Netherlands). Both inter- and Intra-assay coefficients of variations of the assays were <5%.
Using a simple and valid spectrophotometric method, we measured serum NOx concentration; this method was developed by Miranda et al. and validated in our laboratory [Citation26,Citation27]. Inter- and Intra-assay coefficients of variations of the measurements were 5.2% and 4.4%, respectively; the sensitivity of the assay was 2.0 μmol/L and its recovery was 93 ± 1.5% [Citation28].
Definition of terms and outcome
Type 2 diabetes was defined as using anti-diabetic drugs, FSG ≥7.0 mmol/L or 2–h SG ≥11.1 mmol/L [Citation29]. Hypertension was defined as SBP ≥140 mm Hg or DBP ≥90 mmHg, or self-reported taking blood pressure lowering medication [Citation30].
Fatal and non-fatal chronic heart disease (CHD) events, stroke or cerebrovascular events were considered as CVD outcomes; CHD included cases of definite myocardial infarction (MI) diagnosed by electrocardiogram (ECG) and biomarkers, probable MI (positive ECG findings plus cardiac symptoms or signs and biomarkers showing negative or equivocal results), unstable angina pectoris (new cardiac symptoms or changing symptom patterns and positive ECG findings with normal biomarkers) and angiographic proven CHD. Information of all-cause and CVD mortality were collected by an authorized local physician from the hospital or the death certificate. Data collected were evaluated by an outcome committee (Cohort Outcome Panel) consisting of a principal investigator, an internist, an endocrinologist, a cardiologist, an epidemiologist, and the physician who collected the outcome data [Citation31]. Total number of CVD- and all-cause mortalities were 52 (38 men and 14 women) and 124 (77 men and 47 women), respectively.
Statistical analysis
The mean (±SD) values and the proportions of baseline characteristics of the participants with and without the events, were compared using the independent sample t test or Chi square test, respectively.
To determine cross-sectional correlates of circulating NOx, and explore clinical variables which may explain its inter-individual variation, a multivariable linear regression was conducted. The candidate variables included in this analysis were serum creatinine, WC, TG to HDL-C ratio, SBP, DBP and FSG. To determine the cut-off points of serum NOx for predicting all-cause and CVD mortality, the receiver operator characteristic (ROC) curve analysis was used with an estimation of the variable’s sensitivity and specificity. The cut-off points of the variable was assessed by the maximum value of sensitivity + specificity-1 (Youden index), which has been recommended that it is preferable for finding the optimal cut-off points, because it is clinically translated to maximizing correct classification and minimizing misclassification rates [Citation32]. We also calculated the predictive power, assessed using the area under curve (AUC) of serum NOx levels for all-cause and CVD mortality, using logistic regression analysis.
Multivariate Cox proportional hazard models were used to estimate the hazard ratios (HRs) with 95% confidence intervals (95% CIs) of all-cause and CVD mortality below and above the defined optimal cut-off points for serum NOx. Survival time of the study was the time from entrance to the study to the date of the first related event. The censoring time of an individual was the time from date of entry into the study until lost to follow-up, death or the study end (20 March 2014).
Potential confounding variables adjusted in the multivariate model was age (years), sex (male/female), body mass index (BMI) (kg/m2), smoking (past or current/never), HTN (yes/no), type 2 diabetes (yes/no), and history of CVD (yes/no). Potential confounding variables were selected according to previous reports regarding the major known risk factors for all-cause and CVD mortality in our population [Citation31]. The proportionality of the multivariable Cox models were evaluated using Schoenfeld’s global test of residuals and all proportionality hypotheses were appropriate.
We also used Kaplan-Meier plots to graphically show the survival time for cut-off point of serum NOx.
All analyses were performed using IBM SPSS for Windows version 20 and STATA version 12 SE (StataCorp LP, TX, USA), with a two-tailed p value <.05 being considered significant.
Results
Mean age of participants was 44.5 ± 16.0 years at baseline and 40.2% were male. Median (inter-quartile range) of serum NOx levels was 25.0 µmol/L (19.0–37.0), at baseline. In a median of 7.7 years of follow-up, the incidence rate (95% CI) of CVD and all-cause mortality were 1.97 (1.50–2.59) and 4.71 (3.95–5.61) per/1000 person years, respectively. Baseline characteristics of the participants across serum NOx levels are presented in . Compared to participants who had lower levels of serum NOx at baseline, those with higher circulating NOx had also higher BMI, WC, SBP, FSG, TG to HDL-C ratio, and a higher rate of diabetes, HTN, and history of CVD baseline (p for all <.01).
Table 1. Baseline characteristics of the participants across serum NOx levels.
compares baseline characteristics in subjects with and without events. Compared with the rest of the cohort, participants with all-cause and CVD mortality were more likely to be older, and had higher waist circumference, SBP, FSG, serum NOx levels, and a higher rate of diabetes, HTN, and history of CVD at baseline (p for all <.05). A lower level of physical activity and higher DBP was also observed in participants with CVD mortality compared to the rest of cohort (p for all <.05).
Table 2. Baseline characteristics of the participants: Tehran lipid and glucose study 2006–2008 to 2014.
Circulating NOx was positively related to serum creatinine, WC, TG to HDL-C ratio, and FSG (p for all <.05). These clinical variables explained 3.3% of the inter-individual variation of serum NOx (data not shown).
Optimal cut-off values of serum NOx levels for predicting CVD and all-cause mortality, as well as sensitivity, specificity, and AUC (P value) are presented in . As shown, the optimal cut-off points of serum NOx levels for predicting CVD and all-cause mortality were 30.5 and 32.5 µmol/L, respectively. The AUC and 95% CI of serum NOx for predicting CVD and all-cause mortality in unadjusted-logistic regression model was 0.59 (0.54–0.64, p = .001) and 0.60 (0.52–0.68, p = .012), respectively.
Table 3. Optimal cut-off values of serum NOx levels (μmol/L) for predicting CVD and all-cause mortality.
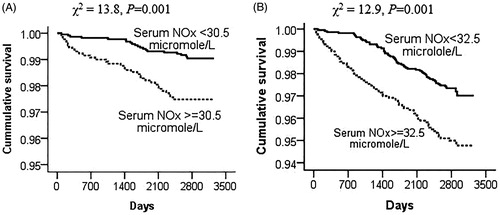
As shown in Kaplan-Meier plots (), the incidence of CVD mortality (61.5% vs. 38.5%, p = .001) and death (52.4 vs. 47.6%, p = .001) was higher in the participants with serum NOx levels ≥30.5 and 32.5 µmol/L, respectively.
Figure 1. Kaplan-Meier curves to estimate the cumulative incidence of events (%) according to serum NOx optimal cut-off values for (A) CVD mortality and (B) all-cause mortality. The log-rank P value of 0.001 represents differences between values under and over the cut-off values.
Hazard ratios and 95% CIs of CVD and all-cause mortality across the optimal cut-off values of serum NOx levels, are shown in . A significant increased risk of CVD mortality (HR = 2.75, 95% CI = 1.57–4.82, HR = 1.98, 95% CI = 1.10–3.58, in the crude and adjusted model, respectively) was observed in the participants who had baseline serum NOx levels ≥30.5 µmol/L. Hazard ratios (95% CI) of all-cause mortality in serum NOx levels ≥32.5 µmol/L were 2.03 (1.43–2.88) and 1.52 (1.05–2.21) in the crude and adjusted model, respectively.
Table 4. Hazard ratios and 95% confidence intervals of CVD and all-cause mortality across the optimal cut-off values of serum NOx levels (μmol/L).
Discussion
In the presence of potent traditional risk factors, including HTN, history of CVD, and type 2 diabetes, elevated serum NOx levels more than 30.5 and 32.5 µmol/L increased risk of CVD and all-cause mortality by 98% (p = .023) and 52% (p = .028); these findings suggested that high serum NOx levels may play as an aggravating factor contributing to the risk of hard clinical end points in a general population.
Mechanistically, NO however has critical roles in cardiovascular homeostasis within the physiological levels, overproduction of NO through compensatory pathways in the pathological status, oxidative stress or inflammatory processes, seems to be a predictor of cardiometabolic disorders likes obesity, type 2 diabetes, hypertension, chronic kidney disease, metabolic syndrome and CVD outcomes [Citation20–22,Citation33–35]. Although current knowledge is rather confusing, overproduction of NO is mainly attributed to pathological elevation of iNOS expression and its functional activity [Citation36,Citation37].
Although both cross-sectional and prospective associations of serum NOx with cardiometabolic outcomes have been investigated in previous population-based studies [Citation20–22,Citation28,Citation33–35,Citation38], limited document are available relating to prediction of mortality according to circulating NO metabolites. Our findings in this study seems to be compatible with three existing reports. For the first time, Osawa et al. discussed that serum NOx levels may be a new clinical biomarker of survival in elderly patients, and introduced it as useful prognostic marker with efficacy almost equal to that of albumin [Citation39]; this claim was subjected to an increased risk of mortality in relation to circulating NOx (OR = 1.03, 95% CI = 1.01–1.54, per 1 µmol/L increase in serum NOx), in an elderly population following of a 3-year period [Citation39]. Gumanova et al., in a 3-year follow-up of elderly people reported a non-significant 51% (HR = 1.51, 95% CI = 0.92–2.48) increased risk of all-cause mortality in relation to increased serum NOx levels; in this study, an elevated serum NOx levels was accompanied by increased risk of CVD mortality (HR = 2.10, 95% CI = 1.06–4.15), in the presence of the well-known CVD risk factors [Citation17]. In the Framingham Offspring Study, HRs (95% CIs) of all-cause mortality per 1-unit increase in Ln-transformed serum nitrate (NO3) were 1.32 (1.14–1.52) and 1.21 (1.04–1.40) following 17.3 years, in age- and sex-adjusted and fully-adjusted model, respectively [Citation40]. In this analysis, after further adjustment for estimated glomerular filtration rate (eGFR), as a measure of renal function, Mass et al. reported a weakened borderline association between circulating NO3 and outcome; due to lack of significant relation between serum NO3 and CVD events in this community, the authors discussed that the observed association of NO3 and mortality might at least in part be mediated by renal function [Citation40]. The positive observed association between circulating NOx and inflammatory markers like C reactive protein (CRP) in these populations [Citation17,Citation39,Citation40] may imply on inflammatory-induced and iNOS-originated elevation of serum NOx. The NO-mortality relation however was essentially independent of the major known inflammatory markers including CRP, interlukine-6, and tumor necrosis factor-α, in these studies [Citation17,Citation39,Citation40].
Regardless of our previous report [Citation19], the normal range or reference values of serum NOx have not yet been reported and similarly, we did not find a definite cut-off point of serum NOx levels for clinical outcomes in other populations. Compared to our determined cut-offs of serum NOx for CVD and all-cause mortality (30.5 and 32.5 µmol/L), a considerable higher level of circulating NOx has been reported in relation to hard clinical end points in previous investigations. An increased risk of mortality was only observed in serum NOx levels ≥74.6 µmol/L in a Russian population; this value for CVD mortality was in range of 59.3–74.9 µmol/L [Citation17]. In the Framingham Offspring Cohort, higher rate of mortality was observed in serum NO3 levels ≥60.9 µmol/L (IQR = 53.0–76.9) [Citation40].
In determining the relationship between evidence of elevated serum NOx and potential health consequences like mortality, several factors need to be clarified; the source of circulating NO metabolites, including potential involvement of its endogenous iNOS-induced overproduction, its probable accumulation due to renal dysfunction, and exogenous NO3 and nitrite (NO2) exposure, should ideally be considered. Lack of effective measurements for simultaneous analysis of serum, urinary and dietary NO3/NO2 exposure, may therefore be responsible for some inconsistencies observed in the current literature. Although, it most believed that circulating NO metabolites, especially in a fasted state, is an indicator of systemic NO production, under a regular diet, serum NOx may be corresponding to a global measure of NO3/NO2 exposure form both exogenous and endogenous sources [Citation41,Citation42].
Defining of an optimal population-specific cut-off value of serum NOx levels for predicting of hard clinical endpoints like all-cause and CVD mortality, was the main potential strength of this study. This approach provides a decision threshold to dichotomize biomarker levels for providing benchmarks according to the test results [Citation32].
As inherent in any prospective study, a major limitation of this study was probable changes in serum NOx levels and other risk factors during the follow-up period. As such, some degree of misclassification might have occurred, leading to biased estimated HRs toward the null. Lack of dietary information regarding habitual intakes of NO3 and NO2 may also be considered as a limitation of this study; however weak correlations between serum NO3/NO2 and their dietary values, which has been previously reported in a sub-sample of our population, indicating that fasting serum NOx values is relatively independent of NO3/NO2 dietary exposure.
Conclusion
To our knowledge this is the first attempt to determine the optimal cut-off values of serum NOx for prediction of CVD and all-cause mortality, using a prospective population-based approach. In conclusion, our findings imply that increased serum NOx, most probably due to endogenous overproduction of NO, may be considered as an independent factor contributing to CVD and all-cause mortality events. With respect to these findings, serum NOx may be suggested as a novel predictor of future risk of cardiovascular mortality and death. Following general characteristics of the measurement method, including non-invasive, rapid and inexpensive, and easily measured, serum NOx seems a good predictor of hard clinical endpoints.
Compliance with ethical standards
The study protocol was accordance to the 1964 Helsinki declaration and approved by the ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (IR.SBMU.ENDOCRINE.REC.1396.487).
Informed consent
Written informed consents were obtained from all participants included in the study.
Acknowledgements
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- World Health Organization. Cardiovascular diseases (CVDs) fact sheet 2017 [cited 2017 Dec 31]. Available from: www.who.int/mediacentre/factsheets/fs317/en
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
- Ge Y, Wang TJ. Identifying novel biomarkers for cardiovascular disease risk prediction. J Intern Med. 2012;272:430–439.
- Hozawa A, Folsom AR, Sharrett AR, et al. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects–Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579.
- Ajani UA, Ford ES. Has the risk for coronary heart disease changed among U.S. adults? J Am Coll Cardiol. 2006;48:1177–1182.
- Ghasemi A, Zahediasl S. Is nitric oxide a hormone? Iranian Biomed J. 2011;15:59–65.
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167.
- Knott AB, Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes Obes Metab. 2010;12:126–133.
- Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1–9.
- Masha A, Dinatale S, Allasia S, et al. Role of the decreased nitric oxide bioavailability in the vascular complications of diabetes mellitus. CPB. 2011;12:1354–1363.
- Stoclet JC, Muller B, Andriantsitohaina R, et al. Overproduction of nitric oxide in pathophysiology of blood vessels. Biochem Biokhimiia. 1998;63:826–832.
- Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143.
- Hewala TI, Abd El-Moneim NA, Ebied SA, et al. Diagnostic and prognostic value of serum nitric oxide, tumor necrosis factor-alpha, basic fibroblast growth factor and copper as angiogenic markers in premenopausal breast cancer patients: a case-control study. Brit J Biomed Sci. 2010;67:167–176.
- Muto S, Takagi H, Owada Y, et al. Serum nitric oxide as a predictive biomarker for bevacizumab in non-small cell lung cancer patients. Anticancer Res. 2017;37:3169–3174.
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. European Heart J. 2012;33:829–837d.
- Feng Q, Lu X, Jones DL, et al. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation. 2001;104:700–704.
- Gumanova NG, Deev AD, Zhang W, et al. Serum nitrite and nitrate levels, NOx, can predict cardiovascular mortality in the elderly in a 3-year follow-up study. BioFactors (Oxford, England). 2017;43:82–89.
- Beberashvili I, Sinuani I, Azar A, et al. Increased basal nitric oxide amplifies the association of inflammation with all-cause and cardiovascular mortality in prevalent hemodialysis patients. Int Urol Nephrol. 2013;45:1703–1713.
- Ghasemi A, Zahediasl S, Azizi F. Reference values for serum nitric oxide metabolites in an adult population. Clin Biochem. 2010;43:89–94.
- Bahadoran Z, Mirmiran P, Ghasemi A, et al. Serum nitric oxide metabolites are associated with the risk of hypertriglyceridemic-waist phenotype in women: Tehran Lipid and Glucose Study. Nitric Oxide. 2015;50:52–57.
- Bahadoran Z, Mirmiran P, Tahmasebi Nejad Z, et al. Serum nitric oxide is associated with the risk of chronic kidney disease in women: Tehran lipid and glucose study. Scand J Clin Lab Invest. 2016;76:304–308.
- Hadaegh F, Asgari S, Bozorgmanesh M, et al. Added value of total serum nitrate/nitrite for prediction of cardiovascular disease in middle east caucasian residents in Tehran. Nitric Oxide. 2016;54:60–66.
- Azizi F, Rahmani M, Emami H, et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Soz Praventivmed. 2002;47:408–426.
- Azizi F, Ghanbarian A, Momenan AA, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:5.
- Momenan AA, Delshad M, Sarbazi N, et al. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15:279–282.
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71.
- Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. JMSR. 2007;2:29–32.
- Ghasemi A, Zahediasl S, Azizi F. Elevated nitric oxide metabolites are associated with obesity in women. Arch Iran Med. 2013;16:521–525.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2014;37:S14–S80.
- Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Sardarinia M, Akbarpour S, Lotfaliany M, et al. Risk factors for incidence of cardiovascular diseases and all-cause mortality in a Middle Eastern Population over a decade follow-up: Tehran Lipid and glucose study. PLoS One. 2016;11:e0167623.
- Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675.
- Ghasemi A, Zahediasl S, Azimzadeh I, et al. Increased serum nitric oxide metabolites in dysglycaemia. Ann Hum Biol. 2011;38:577–582.
- Ghasemi A, Zahediasl S, Azizi F. High serum nitric oxide metabolites and incident metabolic syndrome. Scand J Clin Lab Invest. 2012;72:523–530.
- Ghasemi A, Zahediasl S, Syedmoradi L, et al. Association between serum nitric oxide metabolites and hypertension in a general population. Int Angiol. 2011;30:380–387.
- Shah AM. Inducible nitric oxide synthase and cardiovascular disease. Cardiovasc Res. 2000;45:148–155.
- Viaro F, Nobre F, Evora PR. Expression of nitric oxide synthases in the pathophysiology of cardiovascular diseases. Arquivos Brasileiros de Cardiologia. 2000;74:380–393.
- Zahedi Asl S, Ghasemi A, Azizi F. Serum nitric oxide metabolites in subjects with metabolic syndrome. Clin Biochem. 2008;41:1342–1347.
- Osawa M, Hayashi T, Nomura H, et al. Nitric oxide (NO) is a new clinical biomarker of survival in the elderly patients and its efficacy might be nearly equal to albumin. Nitric Oxide. 2007;16:157–163.
- Maas R, Xanthakis V, Goen T, et al. Plasma nitrate and incidence of cardiovascular disease and all-cause mortality in the community: The Framingham Offspring Study. J Am Heart Assoc. 2017;6:e006224.
- Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400.
- Gilchrist M, Shore A. Inorganic nitrate: marker or mediator of mortality? J Am Heart Assoc. 2017;6:e007782.
