Abstract
Objective. We aimed to summarize the evidence from observational studies examining the risk factors of the incidence of mediastinitis in open heart surgery. Design. The study was a systematic review and meta-analysis of cohorts and case-control studies. Material and methods. We searched the literature and 74 studies with at least one risk factor were identified. Both fixed and random effects models were used. Heterogeneity between studies was examined by subgroup and meta-regression analysis. Publication bias or small study effects were evaluated and corrected by limit meta-analysis. Results. When correcting for small study effect, presence of obesity as estimated from 43 studies had Odds Ratio OR = 2.26. (95% CI: 2.17–2.36). This risk was increasing with decreasing latitude of study place. Presence of diabetes mellitus from 63 studies carried an OR = 1.90 (95% CI: 1.59–2.27). Presence of Chronic Obstructive Pulmonary Disease (COPD) from 30 studies had an OR = 2.59 (95% CI: 2.22–2.85). Presence of bilateral intramammary graft (BIMA) from 23 studies carried an OR = 2.54 (95% CI: 2.07–3.13). This risk was increasing with increasing frequency of female patients in the study population. Conclusion. Evidence from this study showed the robustness of the risk factors in the pathogenesis of mediastinitis. Preventive measures can be implemented for reducing obesity, especially in lower latitude countries. Furthermore, it is mandatory to monitor perioperative hyperglycemias with continuous insulin infusion. Use of skeletonized BIMA carries higher risk of mediastinitis especially in female patients without evidence of beneficial effect on survival for the time being.
Introduction
The reported incidence of mediastinitis after coronary artery bypasses grafting (CABG) is 0.4 to 4%. Mediastinitis is associated with increased morbidity, mortality and cost. The traditional management consists of reoperation with debridement and drainage, followed by prolonged hospitalisation for antibiotic therapy. Early or in- hospital mortality is reported to vary between 10 and 47%, and there is still an excess risk of mortality after 10 years of follow up [Citation1]. Several risk factors have been identified in the literature, however there are some inconsistencies regarding the role of individual factors in the pathogenesis of sternal infection. Most factors include diabetes mellitus, obesity, chronic obstructive pulmonary disease (COPD) and the presence of bilateral intra mammary graft (BIMA). Also the presence of mediastinitis in open heart surgery carries an increasing risk of obstruction of intra mammary graft. The incidence and microbiology of mediastinitis are dependent of the latitude of the country [Citation2,Citation3] and age of the patient.
The aim of this study is to identify and quantify risk factors of mediastinitis, and to evaluate their robustness, and to pinpoint the influence of patient and study related characteristics on the incidence of mediastinitis in the medical literature.
Methods
Literature search
A qualified medical librarian was consulted at the Medical Library, Oslo University Hospital. We searched the Cochran Central Register of controlled trials (1970–2019), Medline/PubMed (1966–2019) and Embase (1980–2019). We used a combination of keywords, in the search procedure. The last update for research was done on January 2th, 2019. No limitation on language was considered ().
Studies selection
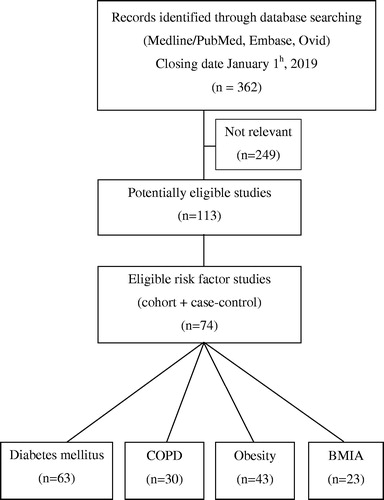
We included cohort studies, (prospective and retrospective), and case control studies on patients with open heart surgery (CABG and valvular heart replacement) and with at least one risk factor Diabetes, Obesity, COPD or BIMA. Two reviewers independently evaluated reports for eligibility. Disagreements were resolved by discussion. Finally, 74 studies were considered eligible for risk factors study [Citation1,Citation4–76]. Sixty three studies for the association diabetes and incidence of mediastinitis [Citation1,Citation4–14,Citation16,Citation17,Citation19–27,Citation30,Citation31,Citation33–37, Citation39–42,Citation44–47,Citation49–62,Citation64–71,Citation74,Citation75]. Forty three studies were selected for studying the association between presence of obesity and incidence of mediastinitis, [Citation1,Citation4,Citation5,Citation7–10,Citation12–14, Citation16–21,Citation23,Citation25,Citation26,Citation30–32,Citation34–36,Citation38,Citation40,Citation44–46,Citation48,Citation50,Citation53,Citation59–61, Citation66–68,Citation71,Citation73,Citation75]. Also 30 studies for the association between presence of COPD and incidence of mediastinitis [Citation1,Citation5,Citation7,Citation9,Citation12,Citation13,Citation16,Citation17,Citation20,Citation21,Citation23,Citation27,Citation29,Citation30,Citation34,Citation37,Citation38,Citation43,Citation50,Citation52–55, Citation59–61,Citation64,Citation66,Citation70,Citation73], and 23 for the association between presence of BIMA and incidence of mediastinitis [Citation5,Citation8,Citation9,Citation11,Citation15,Citation17,Citation20,Citation24,Citation28,Citation36,Citation44,Citation52,Citation54,Citation56,Citation60,Citation61,Citation63,Citation67,Citation70,Citation72,Citation74, Citation76,Citation77].
Quality assessment of the cohort and case-control studies: the component approach
Two of the reviewers independently assessed the studies. For the purpose of critically appraising case-control and cohort studies we developed a check-list based on recommendations by experienced investigators [Citation78].The poor reporting on important methodological details may be due to the fact that most papers were published before the STROBE statements [Citation79]. Sanderson et al [Citation80] have warned for the use of scale. Scales resulting in numerical summary of quality scores can introduce a bias when assessing study quality [Citation81].
Data abstraction
Data regarding publication status, study design, patient-related characteristics, outcome methods, result and funding were extracted in duplicate on a standardized form according to an a priori protocol. Patient-related variables included mean age of the cohort, frequency of female gender, and frequency of CABG operation. The study-level variables included the nature of the cohort, prospective versus retrospective, the use of case-control versus cohort in the risk factors estimation, use of multivariate methods to control for confounders, latitude and longitude of the place of the study.
Endpoints considered
The primary endpoint was the incidence of mediastinitis. The diagnosis of post sternotomy mediastinitis was based upon the criteria established by the Centres for Disease Control and Prevention (CDC).
Risk Factors of mediastinitis as exposition were presence of obesity (BMI > 30), diabetes mellitus, Chronic Obstructive Pulmonary Disease (COPD) and Bilateral Intra Mammary Graft operation (BIMA) as compared to Single Intra Mammary Graft.
Quantitative data synthesis
Statistical pooling
Fixed and random effects model analyses were considered to calculate an overall effect. The DerSimonian Laird estimate [Citation82] is used in the random effects model. The risk factors of mediastinitis (diabetes, obesity, COPD, and BIMA) were quantified by the OR and its 95% confidence intervals. The information required from each study is an appropriate measure of odds ratio, OR, and its variance, V, which can be calculated if the lower and upper confidence limits ORL and ORU are given.
Sources of heterogeneity, evaluation and quantification
Statistical heterogeneity among studies was assessed with Cochran’s Q test. The magnitude of heterogeneity was evaluated by the I2 statistics which describes the proportion of total variation due to heterogeneity rather than chance. In order to investigate possible sources of heterogeneity, two different methods were used: subgroup analyses and meta-regression.
Evaluation of selection bias or small study effect
Publication bias is known to occur in meta-analyses. In order to assess potential publication bias or small-study effect we used three well established test of small study effect or selection bias; Begg and Mazumdar Rank correlation [Citation83], Egger’s test of asymmetry [Citation84], and Test of moment for asymmetry [Citation85].
Adjusting for small study effect (publication bias) using the limit Meta-analysis method
A popular method for detecting and adjusting publication bias or small study effect is the “trim and fill” method [Citation86]. This method is known to perform poorly in the presence of substantial between-study heterogeneity [Citation87,Citation88]. A regression-based approach is a promising alternative, namely the method of limit meta-analysis [Citation89]. This method is robust against heterogeneity [Citation90]. Power considerations in meta-analysis and meta-regression were following the recommendations of Borenstein M et al [Citation91].
All statistical analyses were performed with R–Package–Meta [Citation92]. We have followed the PRISMA guidelines for meta-analyses and systematic reviews of observational studies [Citation93] in the present study.
Results
Trial flow
After identifying 362 references, many studies were excluded due to irrelevant content and duplicate publications, leaving 63 potentially eligible studies for diabetes, 43 for obesity, 30 for COPD, and 23 for BIMA ().
Risk factors of mediastinitis
Obesity
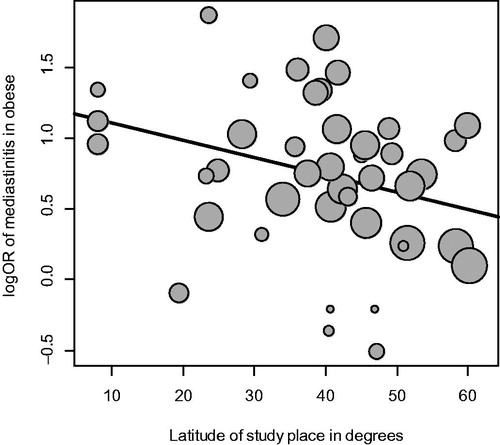
The summarized pooled estimate of the association between obesity and incidence of mediastinitis in the 43 studies using the random effect model was OR = 2.16 95% CI (1.79–2.60) with a substantial heterogeneity 92.0% (). The funnel plot visually showed evidence of bias and small study effect. This was verified using Begg and Mazumdar Rank and confirmed by the Egger’s test. To control for selection bias and small study effect we considered the limit meta-analysis method because of substantial heterogeneity. The adjusted estimate was OR = 2.26 95% CI (2.17–2.30).One important study-level related variable identified was latitude () (), with increasing effect of obesity on the incidence of mediastinitis with decreasing latitude of study place. The amount of heterogeneity accounted for latitude was 56.7%
Table 1. Pooled estimate of total or for the association between risk factors and incidence of mediastinitis with stratification on study level variables using the random effect model.
Table 2. Estimate of the mixed-effect regression model between risk factors and incidence of mediastinitis, and the different study-level and patient-level variables.
Diabetes mellitus
The summarized pooled estimate of the association between diabetes mellitus and incidence of mediastinitis in the 63 studies using the random effect model was OR = 2.31, 95% CI (2.14–2.49) with a little heterogeneity 2.3% (). The funnel plot visually showed evidence of bias and small study effect. This was confirmed by the Egger’s test, and the test of method. To control for selection bias and small study effect we considered the limit meta-analysis method because of substantial heterogeneity. The adjusted estimate was 95% CI OR = 1.90, (1.59–2.27). None of the patient level and study level covariates showed significance in the mixed effect model ().
Chronic obstructive pulmonary disease (COPD)
The summarized pooled estimate of the association between COPD and incidence of mediastinitis in the 30 studies using the random effect model was OR = 2.53, 95% CI (2.11–3.01) with a moderate heterogeneity 35.2% (). The funnel plot visually did not show evidence of selection bias or small study effect. This was verified using the three different tests of selection bias. No limit meta-analysis method was performed. None of the patient level and study level covariate showed significance using the mixed effect model ().
Bilateral intra mammary grafting (BIMA)
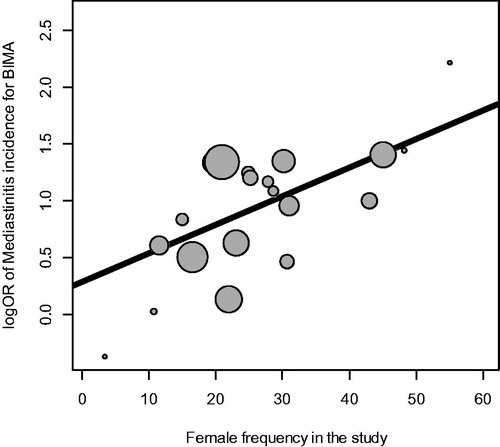
The summarized pooled estimate of the association between BIMA and incidence of mediastinitis in the 23 studies using the random effects model was OR = 2.49, 95% CI (2.05–3.03) with a substantial heterogeneity 39.4% (). No small study effect was demonstrated. No adjustment by limit meta-analysis was necessary. One important patient-level related variable identified was female gender () () with increasing risk of mediastinitis with increasing frequency of female gender in the study. The amount of heterogeneity accounted for female gender was 58.7%
Discussion
Our systematic review and meta-analysis of risk factors of mediastinitis revealed robust risk factors. Obesity as an independent risk factor was decreasing in magnitude with increasing latitude of the study place. Also BIMA as a risk factor of mediastinitis was stronger in female as compared to male. There was substantial heterogeneity that was explained by some patients and study related characteristics and was investigated using metaregression. There was a presence of small study effect/publication bias and this was pinpointed and corrected by limit meta-analysis and not the trim and fill method because of substantial heterogeneity.
Strengths and limitations
Our review was based on a broad literature search, and it seems unlikely that we missed relevant studies [Citation94]. The study selection, data extraction, and data assessment were done by two authors, to minimize bias and transcription errors [Citation95]. The major limitations of our study were the quality of the studies, mainly the retrospective nature of the cohort and the non-random nature of the control group in case-control design, with poor control for confounders and no investigations of effect modification in both cohort and case control studies. There was variability in the diagnosis of obesity and no specification of the nature of diabetes mellitus as diabetes I or II in some studies. There was a possibility of misclassification of the risk factors between patients with mediastinitis and control or non-mediastinitis, but this could have been a non-differential misclassification diluting the magnitude of the risk factors. Small-study effect and selection bias represented a problem in our systematic review. This was corrected using the method of limit meta-analysis, and heterogeneity was investigated and quantified. The majority of studies had no a priori power analysis and some of them were power deficient.
Obesity as an independent risk factor of mediastinitis
The estimation of OR controlling for small study effect was 2.26, 95% CI (2.17–2.30) with substantial heterogeneity. This effect was robust to sensitivity analysis. As underlined by Falagas ME et al [Citation96] the incidence of nosocomial infections in overweight and obese patients is increased compared with regular weight patients, usually because of prolonged duration of stay in hospitals, thus increasing their risk of infections. On the other hand, obesity was found to be associated with staphylococcus aureus nasal carriage, when adjusted for other variables. An aggressive regimen to lower obesity could be implemented before open heart surgery, especially in low latitude countries. On the other hand we pinpointed in our previous systematic review and meta-analysis [Citation3] that latitude of study place is associated with temperature regulated bacterial virulence and is a marker of surgical local practice.
Diabetes mellitus as independent risk factor of mediastinitis
The estimation of OR controlling for small study effect was 1.90, 95% CI (1.59–2.27) with minimal heterogeneity. This result was robust when performing a sensitivity analysis.
This increased infection risk in diabetic patients can be imputed in part to physiologic modifications precipitated by deficient glucose control as underlined by Talbot TR [Citation89]. Over time, chronic hyperglycaemia results in small vessel vasculopathy, contribute to local tissue hypoxia and ischemia, possibly diminished penetration of antibiotics to the surgical field for phagocytosis of invading bacteria. All these components contribute to an increased risk of mediastinitis in diabetic patients.
There is a gradient effect between level of perioperative serum glucose level and incidence of surgical site infection in open heart surgery [Citation97]. Nowadays we have evidence for the benefit of controlling perioperative and postoperative glucose levels in both diabetic patients and hyperglycaemic non diabetic patient by using continuous insulin infusion (CII) according to the Portland protocol [Citation98].
Chronic obstructive pulmonary disease (COPD) as independent risk factor of mediastinitis
The estimation of OR was 2.53, 95% CI (2.11–3.01) with heterogeneity. This result was robust when performing a sensitivity analysis.
As underlined by Wilson and Sethi [Citation99,Citation100] epidemiological cohorts of acute development of COPD are difficult because of the heterogeneous nature of the disease, the part of bacterial infection, and consequently the usage of antibiotics is complex. Some COPD patients are constantly colonized by bacteria between aggravations periods. This could explain the reason why those patients get mediastinitis.
Bilateral ima grafting (BIMA) as independent risk factor of mediastinitis
The estimation of OR was 2.49, 95% CI (2.05–3.03) from 23 studies with heterogeneity. This result was robust when performing a sensitivity analysis and was confirmed by the ART (Arterial Revascularization Trial) [Citation77]. The incidence of Deep sternal wound complication was 3.5% for BIMA as compared to 1.9% in SIMA with OR = 1.9, 95% CI (1.20–3.0) p = .005. The pooled incidence from 22 studies was OR = 2.54 95% CI (2.07–3.01) and was included in the 95% confidence interval of the ART trial. Taggart DP et al [Citation101] in an overview and meta-analysis underlined that BIMA had a better survival than SIMA (Single IMA grafting). . The 10 years ART trial was recently published [Citation102] showing no efficacy on total mortality and composite end point in an intention to treat analysis. Because of methodological problems it could be considered neutral [Citation103]. And further studies are needed to be conclusive on this topic.
Implications for research
The quality of reporting in the included studies of our meta-analysis was poor. Future studies with cohort or case-control design should adhere to the methodological standards that reduce possible bias, including prospective/retrospective cohorts, blinding of exposed/non exposed groups for outcome assessors, measures to reduce the drop-out rate, and an analysis based on all patients recruited. Also, an a priori power analysis is mandatory. Moreover, reports of studies should adhere to generally established standards of reporting [Citation69].
Implications for practice
Prevention and better treatment of mediastinitis complications must be the major goal in assuming the highest quality of cardiovascular care. Prevention needs to handle the major risk factors such as reducing obesity before surgery via an adequate nutritional and physical exercise program, especially in lower latitude countries. Controlling glycaemia, using the Portland protocol, that has shown its efficacy in decreasing incidence of mediastinitis and other complications. It is mandatory to carefully monitor patients with COPD. The procedure of BIMA (bilateral IMA grafting) represents an increasing risk of mediastinitis especially in female patients. Nowadays we cannot confirm its beneficial effect on survival for the patients.
Disclosure
The authors report no conflicts of interest in this work.
Author contribution
All the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All participated in drafting of the article or revising it critically for important intellectual content, and gave final approval of the version to be published.
Acknowledgements
We would like to thank our Medical Librarian, Marie Isachsen, for assisting with the literature search.
References
- Risnes I, Abdelnoor M, Almdahl SM, et al. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg. 2010;89:1502–1509.
- Risnes I, Abdelnoor M, Ulimoen G, et al. Mediastinitis after coronary artery bypass grafting increases the incidence of left internal mammary artery obstruction. Int Wound J. 2014;11:594–600.
- Abdelnoor M, Vengen ØA, Johansen O, et al. Latitude of the study place and age of the patient are associated with incidence of mediastinitis and microbiology in open-heart surgery: a systematic review and meta-analysis. Clin Epidemiol. 2016;8:151–163.
- Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg. 2004;77:676–683.
- Ahmed D, Cheema FH, Ahmed YI, et al. Incidence and predictors of infection in patients undergoing primary isolated coronary artery bypass grafting: a report from a tertiary care hospital in a developing country. J Cardiovasc Surg (Torino). 2011;52:99–104.
- Alserius T, Anderson RE, Hammar N, et al. Elevated glycosylated haemoglobin (HbA1c) is a risk marker in coronary artery bypass surgery. Scand Cardiovasc J. 2008;42:392–398.
- Sá MP, Figueira ES, Santos CA, et al. Validation of MagedanzSCORE as a predictor of mediastinitis after coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2011;26:386–392.
- Antunes PE, Bernardo JE, Eugénio L, et al. Mediastinitis after aorto-coronary bypass surgery. Eur J Cardiothorac Surg. 1997;12:443–449.
- Bitkover CY, Gårdlund B. Mediastinitis after cardiovascular operations: a case-control study of risk factors. Ann Thorac Surg. 1998;65:36–40.
- Blanchard A, Hurni M, Ruchat P, et al. Incidence of deep and superficial sternal infection after open heart surgery. A ten years retrospective study from 1981 to 1991. Eur J Cardiothorac Surg. 1995;9:153–157.
- Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65:1050–1056.
- Braxton JH, Marrin CA, McGrath PD, et al. 10-year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg 2004;16:70–76.
- Cayci C, Russo M, Cheema FH, et al. Risk analysis of deep sternal wound infections and their impact on long-term survival: a propensity analysis. Ann Plast Surg. 2008;61:294–301.
- Centofanti P, Savia F, La Torre M, et al. A prospective study of prevalence of 60-days postoperative wound infections after cardiac surgery. An updated risk factor analysis. J Cardiovasc Surg (Torino). 2007;48:641–646.
- Danzer D, Christenson JT, Kalangos A, et al. Impact of double internal thoracic artery grafts on long-term outcomes in coronary artery bypass grafting. Tex Heart Inst J. 2001;28:89–95.
- Demmy TL, Park SB, Liebler GA, et al. Recent experience with major sternal wound complications. Ann Thorac Surg. 1990;49:458–462.
- Diez C, Koch D, Kuss O, et al. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg. 2007;2:23.
- Eklund AM, Lyytikäinen O, Klemets P, et al. Mediastinitis after more than 10,000 cardiac surgical procedures. Ann Thorac Surg. 2006;82:1784–1789.
- Fakih MG, Sharma M, Khatib R, et al. Increase in the rate of sternal surgical site infection after coronary artery bypass graft: a marker of higher severity of illness. Infect Control Hosp Epidemiol. 2007;28:655–660.
- Farsky PS, Graner H, Duccini P, et al. Risk factors for sternal wound infections and application of the STS score in coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2011;26:624–629.
- Filsoufi F, Rahmanian PB, Castillo JG, et al. Diabetes is not a risk factor for hospital mortality following contemporary coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2007;6:753–758.
- Garey KW, Kumar N, Dao T, et al. Risk factors for postoperative chest wound infections due to gram-negative bacteria in cardiac surgery patients. J Chemother. 2006;18:402–408.
- Ghotaslou R, Yagoubi AR, Khalili AA, et al. Mediastinitis after cardiac surgery in Madani Heart Center, Tabriz, Iran. Jpn J Infect Dis. 2008;61:318–320.
- Grossi EA, Culliford AT, Krieger KH, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg. 1985;40:214–223.
- Gwely NDN, Khalaf SA, Abol Maaty RA. Deep median sternotomy wound infection after open heart surgery. Egyptian J Surg. 2001;20:742–748.
- Harrington G, Russo P, Spelman D, et al. Surgical-site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2004;25:472–476.
- Hassan M, Smith JM, Engel AM. Predictors and outcomes of sternal wound complications in patients after coronary artery bypass graft surgery. Am Surg. 2006;72:515–520.
- Hazelrigg SR, Wellons HA, Jr, Schneider JA, et al. Wound complications after median sternotomy. Relationship to internal mammary grafting. J Thorac Cardiovasc Surg. 1989;98:1096–1099.
- Hosseinrezaei H, Rafiei H, Amiri M. Incidence and risk factors of sternal wound infection at site of incision after open-heart surgery. J Wound Care. 2012;21:408–411.
- Immer FF, Durrer M, Mühlemann KS, et al. Deep sternal wound infection after cardiac surgery: modality of treatment and outcome. Ann Thorac Surg. 2005;80:957–961.
- Jakob HG, Borneff-Lipp M, Bach A, et al. The endogenous pathway is a major route for deep sternal wound infection. Eur J Cardiothorac Surg. 2000;17:154–160.
- Kim J, Hammar N, Jakobsson K, et al. Obesity and the risk of early and late mortality after coronary artery bypass graft surgery. Am Heart J. 2003;146:555–560.
- Kohli M, Yuan L, Escobar M, et al. A risk index for sternal surgical wound infection after cardiovascular surgery. Infect Control Hosp Epidemiol. 2003;24:17–25.
- Lepelletier D, Perron S, Bizouarn P, et al. Surgical-site infection after cardiac surgery: incidence, microbiology, and risk factors. Infect Control Hosp Epidemiol. 2005;26:466–472.
- Loop FD, Lytle BW, Cosgrove DM, et al. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49:179–186; discussion 186–187.
- Lu JC, Grayson AD, Jha P, et al. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23:943–949.
- Paul M, Raz A, Leibovici L, et al. Sternal wound infection after coronary artery bypass graft surgery: validation of existing risk scores. J Thorac Cardiovasc Surg. 2007;133:397–403.
- Magedanz EH, Bodanese LC, Guaragna JC, et al. Risk score elaboration for mediastinitis after coronary artery bypass grafting. Rev Bras Cir Cardiovasc. 2010;25:154–159.
- Matros E, Aranki SF, Bayer LR, et al. Reduction in incidence of deep sternal wound infections: random or real? J Thorac Cardiovasc Surg. 2010;139:680–685.
- Milano CA, Kesler K, Archibald N, et al. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation 1995;92:2245–2251.
- Muñoz P, Menasalvas A, Bernaldo de Quirós JC, et al. Postsurgical mediastinitis: a case-control study. Clin Infect Dis. 1997;25:1060–1064.
- Nagachinta T, Stephens M, Reitz B, et al. Risk factors for surgical-wound infection following cardiac surgery. J Infect Dis. 1987;156:967–973.
- Newman LS1, Szczukowski LC, Bain RP, et al. Suppurative mediastinitis after open heart surgery. A case control study of risk factors. Chest. 1988;94:546–553.
- Noyez L, van DJ, Mulder J, et al. Sternal wound complications after primary isolated myocardial revascularization: the importance of the post-operative variables. Eur J Cardiothorac Surg. 2001;19:471–476.
- Olsen MA, Lock-Buckley P, Hopkins D, et al. The risk factors for deep and superficial chest surgical-site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg. 2002;124:136–145.
- Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg. 1996;111:1200–1207.
- Parissis H, Al-Alao B, Soo A, et al. Risk analysis and outcome of mediastinal wound and deep mediastinal wound infections with specific emphasis to omental transposition. J Cardiothorac Surg. 2011;6:111.
- Prabhakar G, Haan CK, Peterson ED, et al. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons' database. Ann Thorac Surg. 2002;74:1125–1130; discussion 1130–1131.
- Rahmanian PB1, Adams DH, Castillo JG, et al. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. Ann Thorac Surg. 2010;90:1221–1229.
- Careaga Reyna G, Aguirre Baca GG, Medina Concebida LE, et al. Risk factors for mediastinitis and sternal dehiscence after cardiac surgery. Rev Esp Cardiol. 2006;59:130–135.
- Sachithanandan A, Nanjaiah P, Nightingale P, et al. Deep sternal wound infection requiring revision surgery: impact on mid-term survival following cardiac surgery. Eur J Cardiothorac Surg. 2008;33:673–678.
- Sakamoto H, Fukuda I, Oosaka M, et al. Risk factors and treatment of deep sternal wound infection after cardiac operation. Ann Thorac Cardiovasc Surg. 2003;9:226–232.
- Leung Wai Sang S, Chaturvedi R, Alam A, et al. Preoperative hospital length of stay as a modifiable risk factor for mediastinitis after cardiac surgery. J Cardiothorac Surg. 2013;8:45.
- Steingrimsson S, Gottfredsson M, Kristinsson KG, et al. Deep sternal wound infections following open heart surgery in Iceland: a population-based study. Scand Cardiovasc J. 2008;42:208–213.
- Strecker T, Rösch J, Horch RE, et al. Sternal wound infections following cardiac surgery: risk factor analysis and interdisciplinary treatment. Heart Surg Forum. 2007;10:E366–E371.
- Ståhle E, Tammelin A, Bergström R, et al. Sternal wound complications-incidence, microbiology and risk factors. Eur J Cardiothorac Surg. 1997;11:1146–1153.
- Szabó Z, Håkanson E, Svedjeholm R. Early postoperative outcome and medium-term survival in 540 diabetic and 2239 nondiabetic patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2002;74:712–719.
- Tang GH1, Maganti M, Weisel RD, et al. Prevention and management of deep sternal wound infection. Semin Thorac Cardiovasc Surg. 2004;16:62–69.
- Tiveron MG, Fiorelli AI, Mota EM, et al. Preoperative risk factors for mediastinitis after cardiac surgery: analysis of 2768 patients. Rev Bras Cir Cardiovasc. 2012;27:203–210 [English, Portuguese].
- Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, et al. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127:464–471.
- Trick WE, Scheckler WE, Tokars JI, et al. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119:108–114.
- Upton A, Roberts SA, Milsom P, et al. Staphylococcal post-sternotomy mediastinitis: five year audit. ANZ J Surg. 2005;75:198–203.
- Walkes JC, Earle N, Reardon MJ, et al. Outcomes in single versus bilateral internal thoracic artery grafting in coronary artery bypass surgery. Curr Opin Cardiol. 2002;17:598–601.
- Wang FD, Chang CH. Risk factors of deep sternal wound infections in coronary artery bypass graft surgery. J Cardiovasc Surg (Torino). 2000;41:709–713.
- Wilson SJ, Sexton DJ. Elevated preoperative fasting serum glucose levels increase the risk of postoperative mediastinitis in patients undergoing open heart surgery. Infect Control Hosp Epidemiol. 2003;24:776–778.
- Wouters R, Wellens F, Vanermen H, et al. Sternitis and mediastinitis after coronary artery bypass grafting. Analysis of risk factors. Tex Heart Inst J. 1994;21:183–188.
- Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. Deep vs Superficial Infection. Chest 1996;110:1173–1178.
- Badawy MA, Shammari FA, Aleinati T, et al. Deep sternal wound infection after coronary artery bypass: how to manage? Asian Cardiovasc Thorac Ann. 2014;22:649–654.
- Parada JM, Carreño M, Camacho J, et al. Factores asociados a la aparición de mediastinitis en 2.073 revascularizaciones miocárdicas. Rev Colomb Cardiol. 2014;21:119–124.
- Mohammadi S, Dagenais F, Voisine P, et al. Lessons learned from the use of 1,977 in-situ bilateral internal mammary arteries: a retrospective study. J Cardiothorac Surg. 2014;9:158.
- Gatti G, Dell'Angela L, Barbati G, et al. A predictive scoring system for deep sternal wound infection after bilateral internal thoracic artery grafting. Eur J Cardiothorac Surg. 2016;49:910–917.
- Ruka E, Dagenais F, Mohammadi S, et al. Bilateral mammary artery grafting increases postoperative mediastinitis without survival benefit in obese patients. Eur J Cardiothorac Surg. 2016;50:1188–1195.
- Oliveira FDS, Freitas LDO, Rabelo-Silva ER, et al. Predictors of mediastinitis risk after coronary artery bypass surgery: applicability of score in 1.322 cases. Arq Bras Cardiol. 2017;109:207–212.
- Gatti G, Perrotti A, Reichart D, et al. Glycated hemoglobin and risk of sternal wound infection after isolated coronary surgery. Circ J. 2017;81:36–43.
- Sá M, Ferraz PE, Soares AF, et al. Development and validation of a stratification tool for predicting risk of deep sternal wound infection after coronary artery bypass grafting at a Brazilian Hospital. Braz J Cardiovasc Surg. 2017;32:1–7.
- Vrancic JM, Piccinini F, Camporrotondo M, et al. Bilateral internal thoracic artery grafting increases mediastinitis: myth or fact? Ann Thorac Surg. 2017;103:834–839.
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med. 2016;375: 2540–2549.
- Elwood M. Critical appraisal of epidemiological studies and clinical trials. Oxford: Oxford University Press; 2007.
- Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457.
- Sanderson S, Tatt ID, Higgins J. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676.
- Greenland S, O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Thompson SG, Sharp S. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56: 455–463.
- Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126.
- Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–4562.
- Rücker G, Carpenter JR, Schwarzer G. Detecting and adjusting for small-study effects in meta-analysis. Biom J. 2011; 53:351–368.
- Rücker G, Schwarzer G, Carpenter JR, et al. Treatment-effect estimates adjusted for small-study effects via a limit meta-analysis. Biostatistics 2011;12:122–142.
- Borenstein M, Hegges LV, Higgins JPT, et al. Introduction to meta-analysis. 3rd ed. London, United Kingdom: John Wiley & Sons; 2011.
- Schwarzer G. m. An R package for meta-analysis. R News. 2007;7:40–45.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269.
- Egger M, Juni P, Bartlett C, et al. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7: 1–76.
- Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London, UK: BMJ Publishing Group; 1995.
- Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446.
- Talbot TR. Diabetes mellitus and cardiothoracic surgical site infections. Am J Infect Control. 2005;33:353–359.
- Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–360; discussion 360.
- Wilson R. The role of infection in COPD. Chest. 1998;113:242S–248S.
- Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35:1209–1215.
- Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularization on survival: a systematic review of studies comparing bilateral and single mammary arteries. Lancet. 2001;358:870–875.
- Taggart DP, Benedetto U, Gerry S, et al. Bilateral versus single internal – thoracic artey graft at 10 years. N Engl J Med. 2019;380:437–446.
- Taggart DP. Implications of the 10-year outcomes of the Arterial Revascularization Trial (ART) for multiple arterial grafts during coronary artery bypass graft. Eur J Cardiothorac Surg. 2019. doi:10.1093/ejtcts/ezz174.