Abstract
Objectives. Left atrial fibrosis represents a substrate for atrial fibrillation (AF) and cardioembolic events. White matter hyperintensities (WMH) are commonly found on magnetic resonance imaging (MRI) and are regarded, at least partly as ischemic brain lesions. Aortic excess pressure (excessPTI) represents an extra work performed by the left ventricle and is a new risk metric associated with cardiovascular complications. The aim of our study was to assessed whether there is a correlation between the degree of LA fibrosis, aortic excess pressure, and WMH in patients without a history of atrial fibrillation but the presence of risk factors for cardiovascular complications. Design. Thirty-eight subjects (10, females, 28 males, median age 64 years) with risk factors (hypertension, diabetes, heart failure, vascular disease) but no history of AF were recruited. Left atrial fibrosis and brain WMH were estimated by MRI. Aortic excess pressure was obtained non-invasively. Results. Atrial fibrosis correlated significantly with aortic excess pressure (r = 0.65, p < .0001) and was significantly associated with periventricular white matter lesion volume (r = 0.34, p = .036). In multiple regression analysis, atrial fibrosis and age were positively associated with periventricular white matter lesions, while aortic excess pressure was not quite significant associated with WMH. This model explains the 30% variance in white matter lesions volume observed in the study. Left atrial fibrosis was independently associated with excessPTI but not with age and mean BP. This model explained 42% of the variance in an area of atrial fibrosis. Conclusions. Atrial fibrosis in subjects with cardiovascular risk factors and no history of AF is associated with white matter hyperintensities and aortic excess pressure.
Introduction
Atrial remodeling caused by endothelial dysfunction, impaired left ventricular and atrial mechanical function and progressive fibrosis enhance thrombogenic atrial substrate [Citation1]. Atrial fibrosis, which can be identified by the late gadolinium enhancement cardiac MRI (LGE), increases predisposition to atrial fibrillation (AF) and makes it more resistant to the restoration of sinus rhythm [Citation2]. AF patients are usually older and may additionally suffer from diabetes, hypertension or congestive heart failure. These risk factors also contribute to thromboembolism.
Moreover, temporal resolution observed between an episode of AF and stroke, suggests that other components may be involved. Therefore, the contribution of other mechanisms than AF-derived substrate, in the pathomechanism of stroke and other forms of vascular brain injury has been proposed [Citation3]. The need for a new concept is supported by real-life data indicating that the cause of stroke and ischemic brain lesions remains unidentified in a substantial proportion of affected subjects [Citation4]. It seems plausible that a high degree of atrial fibrosis may represent substrate involved in thrombotic complications irrespective of the dysrhythmia.
White matter hyperintensities (WMH) refer to areas of increased intensity on magnetic resonance imaging (MRI) of the human brain, corresponding to lesions which are mainly caused by demyelination and axonal loss. Such injuries are commonly found on MRI, and they are associated with an increased risk of dementia and cognitive decline [Citation5]. For the majority of WMH, the presumed origin is vascular with microembolization as the most frequent cause in individuals with severe atherosclerosis of aorta, carotid or vertebral arteries or different cardiac arrhythmias [Citation6,Citation7]. Since a high WMH burden is found in patients with atrial flutter or fibrillation, both are considered as the leading causes of such brain lesions [Citation8]. Surprisingly, there very few data on the association between WMH and atrial fibrosis in subjects with clinical risk factors for cardioembolic events but without a history of AF.
Recently, the increased aortic excess pressure has been proposed as an independent risk factor for various cardiovascular complications such as left ventricular hypertrophy, heart failure, premature mortality and even ischemic stroke [Citation9]. It was also demonstrated that excess pressure was higher in the cohort of subjects with white matter lesion [Citation10]. Interestingly, it is currently unknown if there exists an association between the excess pressure, left atrial (LA) fibrosis and volume of white matter hyperintensities.
The aim of our study was to assess whether there is a correlation between the degree of LA fibrosis, the aortic excess pressure, and WMH in patients without a history of atrial flutter/fibrillation but the presence of risk factors for cardiovascular complications.
Material and methods
Subjects were randomly recruited through a local outpatient clinic. The study population consisted of patients without any previous episode or a currently present atrial fibrillation/flutter but with various risk factors for non-ischemic stroke included in CHA2DS2-VASc scale. The presence of vascular disease for the CHA2DS2VASc score was defined as at least one of the following: confirmed peripheral artery disease, previous myocardial infarction or the presence of atherosclerotic plaques in the aorta of large arteries.
The exclusion criteria included: implanted electronic devices, chronic renal disease, liver failure, previous or current stroke or transient ischemic attacks. The University Ethics Committee approved the study protocol and written informed consent was obtained from all participants.
Standard anthropometric measures were collected from all patients. Brachial arterial blood pressure was measured by the oscillometric method (705 IT, Omron Healthcare Co, Ltd, Kyoto, Japan) in the supine position (mean of three repeated measurements), after the 10-minute rest.
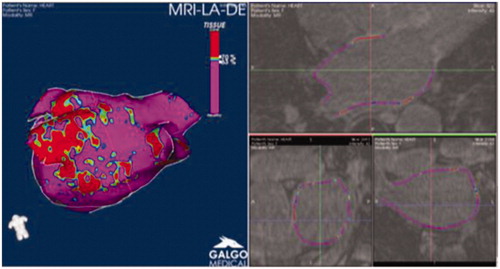
Late gadolinium enhancement cardiac magnetic resonance image acquisition and left atrial fibrosis assessment
Cardiac magnetic resonance imaging was performed on a 3-Tesla LGE-CMR scanner (Magnetom Skyra, Siemens Erlangen Germany) equipped with a 32-channel cardiac coil. 3D-LGE images were acquired from 10 to 20 min after an intravenous bolus injection of 0.2 mmol/kg gadobutrol (Gadovist, Bayer Schering, Germany) in using a 3D free-breathing navigator and an electrocardiogram-gated inversion recovery gradient-echo sequence applied in axial orientation. Typical imaging parameters were the following: TR/TE, 367.04/1.23 ms; flip angle, 22°; voxel size, 0.6 × 0.6 × 1.2 mm; inversion time, 340–360 ms depending on the results of a TI scout scan performed immediately before acquisition; parallel imaging using GRAPPA technique with R = 2; 42 reference lines; acquisition time, 5–10 minutes depending on patient’s heart and breath rate.
Image post-processing and left atrial segmentation
Late gadolinium enhancement cardiac magnetic resonance studies with poor image quality or artifacts were excluded from the analysis. LA wall was segmented using the ADAS image post-processing software (Galgo Medical, Barcelona, Spain), (). The ADAS is a software application developed specifically for the characterization of the left atrium tissue [Citation11–14]. Epicardial and endocardial LA wall contours were manually drawn in each axial plane. To minimize endocardial and epicardial segmentation artifacts, ADAS constructed a mid-myocardial (50% thickness) layer and built a 3D shell that could be edited to ensure that it crossed through the wall. Pulmonary vein at their ostia and the mitral valve were excluded from fibrosis analysis. Pixel signal intensity maps were obtained. Image intensity ratio (IIR) was calculated as the ratio between the signal intensity of each pixel and the mean LA blood pool intensity. Image intensity ratio values were color-coded (fibrosis in red) and projected into the 3D LA shell. Image intensity ratio >1.20 were considered fibrosis. Fibrosis is estimated as cm2 of LA area.
Brain magnetic resonance imaging and segmentation
MRI acquisition was performed on a 1.5 T Siemens Aera scanner (Siemens, Erlangen, Germany) with a 20-channel head-neck coil.
A T1-weighted 3D magnetization prepared rapid acquisition gradient echo sequence (MPRAGE) was obtained in the sagittal plane with TR = 2400 ms; TE = 3.1 ms; flip angle = 8°; TI = 1000 ms; FOV = 241 × 241; acquisition matrix of 256 × 256 × 192 r, giving a reconstructed voxel resolution of 0.9 × 0.9 × 1.0 mm.
For diffusion-weighted imaging a single-shot spin echo planar imaging sequence with 19 slices, slice thickness 1.2 mm, TR = 3300 ms; TE = 110 ms; NEX = 4; acquisition matrix size 192 × 192; FoV = 241; in-plane resolution 0.9 × 0.9 mm and baseline images (b = 0 s/mm2) plus varying diffusion gradient strength along each of three orthogonal directions with b = 500 and 1000 s/mm2 was used. Diffusion trace maps were computed from the isotropic diffusion images and used to estimate ADC values. FLAIR, T2-weighted and TOF hemosequence were also acquired
Segmentation of the T1-weighted 3d brain scans was performed using NeuroQuant (version 2.3, CorTechs Labs Inc, San Diego, CA, USA) which is a fully automated, FDA-cleared tool [Citation15].
Non-invasive assessment of the Central pressure waveform
Brachial blood pressure (BP) was measured by the ascillometric method and the obtained data were automatically used by the device (Colin BPM 7000, Japan) as the reference values for radial tonometry performed with the piezoelectric tonometer. This tonometer was attached to the subject’s wrist was used to record the radial pressure waveform. The acquired signal was transferred in real time to SphygmoCor Mx (AtCor Medical, Australia) for the on-line reconstruction of a pressure waveform for an ascending aorta. Pulse wave analysis commercial software was applied to assess the peripheral and central hemodynamics such as systolic, diastolic blood pressure, augmentation pressure (AP) or augmentation index (AIx@75).
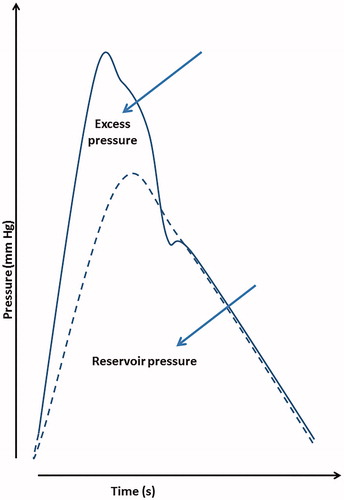
Reservoir–excess pressure model
The reservoir–excess pressure is a model that summarizes the properties of individual arteries ( shows an example of the decomposition of the BP waveform). This model presents measured pressure as the sum of the reservoir pressure and excess pressure. The waveforms measured were obtained using applanation tonometry (SphygmoCor, AtCor Medical, Australia), which generated the radial and corresponding central aortic waveforms as text files. The acquired data were further analyzed numerically using an in-house Python program that was written according to published references [Citation16]. The area under the excess pressure curves (excessPTI = excess pressure–time integral) is presented in units of mmHg·s.
Statistical analysis
All analyses were performed with SPSS (version 23.0, IBM Corp, USA). Due to normal data distribution, all continuous data are reported as mean ± standard deviation (SD). The interrelationships between different parameters were assessed using the parametric Pearson’s correlation. The association between variables was examined using multivariable linear regression model. All tests were considered as statistically significant if p < .05 or of borderline significance if p value was in the range between <.1 and .05.
Results
A total of 38 patients were studied and their baseline characteristics are shown in . The mean age of studied individuals was 65 years, there were 10 women and 28 men. The frequency of at least one risk factor for ischemic stroke according to CHA2DS2-VASc risk score was as follows: five subjects suffered from chronic heart failure, 29 from hypertension, 8 from diabetes, and 29 from vascular disease, and three were older than 75 years. None of the study subjects suffered from atrial fibrillation/flutter as well as stroke or transient ischemic attack.
Table 1. Clinical characteristics of the study subjects.
The association of left atrial fibrosis with white matter lesions and hemodynamic characteristics
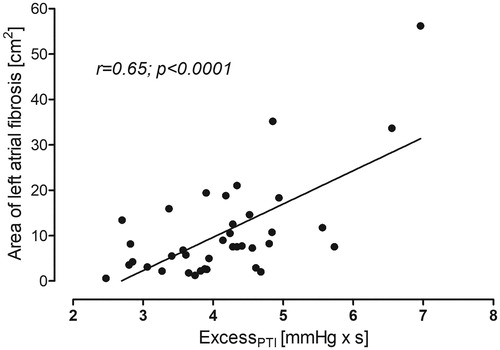
Area of the LA fibrosis correlated weakly (borderline significance, ) with the mean BP, aortic excess pressure (), and the periventricular white matter lesion volume (). Age correlated with periventricular WMH but not with atrial fibrosis, and its correlation with the excessPTI was bordeline (data not shown).
Table 2. Correlation between atrial fibrosis, periventricular white matter lesions, and hemodynamic metrics.
Multivariable analysis for the association of atrial fibrosis, periventricular white matter lesions, and excess pressure
In multiple linear regression area of the LA fibrosis was independently associated with excessPTI but neither with age nor mean BP. This model explained 42% of the variance in the area of LA fibrosis. (, model 1). When AP or AIx@75 was put into the model containing excessPTI, neither augmentation pressure nor augmentation index were associated with the area of LA fibrosis. The presence of AP or AIx@75 did not affect the association of the excessPTI with LA fibrosis (data not shown).
Table 3. Standardized coefficients for multiple linear regression of atrial fibrosis and periventricular white matter lesions volume on independent variables.
The area of LA fibrosis and age were positively associated with periventricular white matter lesions while the excessPTI was weakly (of borderline significance) associated with the WMH (, model 2). This model explains the 30% variance in white matter lesions volume observed in the study. When AP was introduced into the full model, it was not associated with WHM, while excessPTI showed a correlation (data not shown). Including the AIx@75 into the full model did not affect excessPTI association with WMH. Moreover, augmentation index was also not associated with WMH (data not shown).
Discussion
We demonstrate that the area of LA fibrosis correlates with white matter hyperintensities in patients with risk factors for cardiovascular complications. Additionally, we show that the area of LA fibrosis associates with the aortic excess pressure.
Atrial fibrillation is a well-known risk factor for embolic stroke. However, the temporal resolution between episodes of AF and stroke occurrence suggests that other factors independent of dysrhythmia per se are involved [Citation17]. It was also reported that LA fibrosis is associated with thromboembolic events. Despite these findings, it is also possible that LA fibrosis may be regarded only as a marker of increased risk for cardiovascular complication, with or without remote causation of a thromboembolic event.
Surprisingly, there are almost no studies concerning the association between LA fibrosis and brain “vascular” lesions in subjects without atrial fibrillation/flutter. We, therefore, addressed the question of whether any correlation exists between LA fibrosis assessed by MRI and white matter hyperintensities volume as well as hemodynamic markers of increased risk of cardiovascular complication, including ischemic stroke. Our study population consisted of subjects with well-established risk factors for stroke according to the CHA2DS2-VASc scale (apart from atrial fibrillation/flutter). White matter hyperintensities are thought to represent lesions caused (to some extent) by ischemic small vessel disease in the brain. The amount of LA fibrosis correlated positively with periventricular lesion volume, which in turn was also associated with the age of study subjects. In multivariable analysis, both variables (age and LA fibrosis) were independently correlated with the dependent variable. This model explained 30% of the variance in brain periventricular white matter lesion volume observed in the study. Our findings, namely that periventricular white matter lesion are correlated with age are corroborated by others. Nyquist et al. [Citation18] demonstrated that WMH were associated with age, even after controlling for traditional risk factors. Burns et al. [Citation19] showed that periventricular WMH is positively and similarly associated with age in nondemented aging and early-stage Alzheimer disease subjects.
Several studies demonstrated that hemodynamic markers are associated in both prospective and retrospective analysis with increased risk of ischemic stroke. We, therefore, tested the hypothesis of whether LA fibrosis correlates with such a risk metric. High blood pressure is commonly regarded as the most essential factor for increased risk of stroke in both sexes and all age groups [Citation20]. Several studies suggest that BP measured at the periphery does not represent blood pressure measured at the level of the aorta, known as central BP. Moreover, it was also demonstrated that in treated subjects with hypertension similar level of BP at the periphery is not accompanied by a comparable drop in central BP [Citation21]. With the use of pulse wave analysis, several new metrics were derived from central blood pressure, e.g., augmentation pressure, augmentation index or pulse pressure augmentation [Citation22]. More recently, an alternative approach led to the introduction of reservoir and excess pressure components of the estimated central blood pressure [Citation23]. The excess pressure represents the difference between measured BP and reservoir pressure and is an index of the extra work performed by the left ventricle. Moreover, Davies et al. [Citation9] demonstrated that the excess pressure integral was a new indicator of cardiovascular dysfunction and an independent predictor of cardiovascular events and target organ damage in prospective clinical trials. Katulska et al. [Citation10] showed that excess pressure was higher in the cohort of healthy subjects with whiter matter lesion in comparison with those without these findings. Interestingly, in our current study excessPTI, was closely correlated with LA fibrosis which in turn was associated with periventricular WMH. Moreover, the commonly used descriptors of central hemodynamics like augmentation pressure and augmentation index were not associated independently in multivariate analysis with the area of LA fibrosis or WMH (data not shown). Interestingly, Davies et al. [Citation9] showed that in hypertensive patients, the excess pressure but not augmentation pressure was an independent predictor of cardiovascular events.
High blood pressure is the most significant population risk factor for atrial fibrillation and other arrhythmias as well as left ventricular hypertrophy [Citation24]. Brilla et al. [Citation25] demonstrated that lowering BP with lisinopril in subjects with hypertension is associated with regression of myocardial fibrosis. Therefore a plausible explanation for observed finding in our current study is that the excess pressure, as an index of an extra work done by the ventricle in each cardiac cycle, contributes both to the left atrial and ventricular fibrosis [Citation26,Citation27]. The reported here association between the indices of LA fibrosis and white matter hyperintensities proves that such a relationship exists in subjects with cardiovascular risk factors and no atrial fibrillation/flutter.
Study limitation
This study has some limitations. First, relatively few subjects were included, which may affect the strength of the observed association between variables. Second, it is possible that for finding or excluding the correlation between age and the LA fibrosis, a wider age range of study participants should be studied. Third, although the most accurate way to determine the LA fibrosis is a direct pathological examination of atrial biopsy sample, it would be unethical to perform such a study. Fourth, we have not included subjects with a previous or current atrial fibrillation or flutter. We are aware that some patients with asymptomatic atrial fibrillation or flutter in the past might be included, however even long-term monitoring for 7 to 14 days is ineffective if such arrhythmias occur very rarely. Fifth our patients did not have the highest possible risk as for either stroke or TIA. It means that our findings cannot be generalized to whole range of CHA2DS2VASc patients without atrial fibrillation or flutter.
In summary, LA fibrosis in subjects with cardiovascular risk factors and no history of atrial fibrillation/flutter is associated with white matter hyperintensities and aortic excess pressure.
Ethical approval
The University Ethics Committee approved the study protocol and written informed consent was obtained from all participants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fonseca AC, Alves P, Inácio N, et al. Patients with undetermined stroke have increased atrial fibrosis: a cardiac magnetic resonance imaging study stroke. Stroke. 2018;49:734–737.
- King JB, Azadani PN, Suksaranjit P, et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:1311–1321.
- Kamel H, Okin PM, Elkind MS, et al. Atrial Fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900.
- Saver JL, Clinlcal P. Cryptogenic stroke. N Engl J Med. 2016;374:2065–2074.
- Smith CD, Snowdon DA, Wang H, et al. White matter volumes and periventricular white matter hyperintesities in aging and dementia. Neurology. 2000;54:838–842.
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811.
- de Leeuw FE, De Groot JC, Oudkerk M, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke 2000;31:425–429.
- de Leeuw FE, de Groot JC, Oudkerk M, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54:1795–1801.
- Davies JE, Lacy P, Tillin T, et al. Excess pressure integral predicts cardiovascular events independent of other risk factors in the conduit artery functional evaluation substudy of Anglo-Scandinavian Cardiac Outcomes Trial. Hypertension. 2014;64:60–68.
- Katulska K, Wykrętowicz M, Minczykowski A, et al. Aortic excess pressure and arterial stiffness in subjects with subclinical white matter lesions. Int J Cardiol. 2014;172:269–270.
- Benito EM, Carlosena-Remirez A, Guasch E, et al. Left atrial fibrosis quantification by late gadolinium-enhanced magnetic resonance: a new method to standardize the thresholds for reproducibility. Europace. 2017;19:1272–1279.
- Margulescu AD, Nuñez-Garcia M, Alarcón F, et al. Reproducibility and accuracy of late gadolinium enhancement cardiac magnetic resonance measurements for the detection of left atrial fibrosis in patients undergoing atrial fibrillation ablation procedures. EP Europace.2019; 21:724–731.
- Benito EM, Cabanelas N, Nuñez-Garcia M, et al. Preferential regional distribution of atrial fibrosis in posterior wall around left inferior pulmonary vein as identified by late gadolinium enhancement cardiac magnetic resonance in patients with atrial fibrillation. EP Europace. 2018;20:1959–1965.
- den Uijl DW, Cabanelas N, Benito EM, et al. Impact of left atrial volume, sphericity, and fibrosis on the outcome of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:740–746.
- Brewer JB. Fully-automated volumetric MRI with normative ranges: Translation to clinical practice. Behavioral Neurology 2009;21:21–28.
- Parker KH, Alastruey J, Stan GB. Arterial reservoir-excess pressure and ventricular work. Med Biol Eng Comput. 2012; 50:419–424.
- Brambatti M, Connolly SJ, Gold MR, ASSERT Investigators, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–2099.
- Nyquist PA, Bilgel M, Gottesman R, et al. Age differences in periventricular and deep white matter lesions. Neurobiol Aging. 2015;36:1653–1658.
- Burns JM, Church JA, Johnson DK, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–1876.
- Makino Y, Kawano Y, Minami J, et al. Risk of stroke in relation to level of blood pressure and other risk factors in treated hypertensive patients. Stroke. 2000;31:48–52.
- Williams B, Lacy PS, Thom SM, CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225.
- McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725.
- Tyberg JV, Davies JE, Wang Z, et al. Wave intensity analysis and the development of the reservoir-wave approach. Med Biol Eng Comput. 2009;47:221–232.
- Thomas Kahan T, Bergfeldt L. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart. 2005;91:250–256.
- Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393.
- Li EW, McKee-Muir OC, Gilbert PM. Cellular biomechanics in skeletal muscle regeneration. Curr Top Dev Biol. 2018;126:125–176.
- Hall MS, Alisafaei F, Ban E, et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc Natl Acad Sci USA. 2016;113:14043–14048.