Abstract
Objective: Resveratrol (RV) is a polyphenol with antioxidant, anti-inflammatory and cardio-protective properties. Our objective was to investigate whether acute supplementation with high doses of RV would improve flow-mediated dilation (FMD) and oxygen consumption (VO2) kinetics in older coronary artery disease (CAD) patients. Design: We employed a placebo-controlled, single-blind, crossover design in which ten participants (aged 66.6 ± 7.8 years) received either RV or placebo (330 mg, 3× day−1) during three consecutive days plus additional 330 mg in the morning of the fourth day with a seven-day wash-out period in-between. On the fourth day, FMD of the brachial artery and VO2 on-kinetics were determined. Results: RV improved FMD in patients who had undergone coronary artery bypass grafting (CABG; –1.4 vs. 5.0%; p = .004), but not in those who had undergone percutaneous coronary intervention (PCI; 4.2 vs. –0.2%; NS). Conclusion: Acute high dose supplementation with RV improved FMD in patients after CABG surgery but impaired FMD in patients who underwent PCI. The revascularization method-related differential effects of RV may be due to its direct effects on endothelial-dependent dilator responses. Our findings have important implications for personalized treatment and stratification of older CAD patients.
Background
Patients with coronary arterial disease (CAD) commonly present endothelial dysfunction (ED), which is characterized by a compromised capacity of the vessels to dilate in response to endothelium-secreted vasodilators, mainly nitric oxide (NO) [Citation1]. In addition to ED, the presence of atherosclerotic plaques in the coronary arteries may reduce blood flow, and hence oxygen delivery to the myocardium even when cardiac output and systolic blood pressure (BP) are elevated [Citation2]. Atherosclerotic plaques and ED can have a significant impact on skeletal muscle function [Citation3]. Prolonged ATP generation requires an adequate supply of oxygen [Citation4], however, because vessel dilation is impaired, oxygen and nutrient supply to skeletal muscles and the myocardium is not adequate in CAD patients [Citation2]. Consequently, they present lower rates of oxygen consumption (VO2) during exercise [Citation2].
Resveratrol (RV) is a polyphenol with antioxidant, anti-inflammatory, and cardio-protective properties [Citation5]. RV has been shown to reduce oxidative damage in the aorta of aged rats by down-regulating NADPH oxidase while increasing the expression of SIRT1 [Citation6]. In endothelial cells, RV increases the synthesis of NO and protein levels of eNOS [Citation7]. In humans, RV has been shown to enhance insulin sensitivity, reduce BP and improve FMD in obese and type 2 diabetic men [Citation8]. However, other studies have reported that RV blunts the positive effects of regular exercise in healthy young [Citation9] and elderly men [Citation10]. Considering that the effects of RV on endothelial function and VO2 may particularly occur in conditions with elevated systemic inflammation and oxidative stress, we reasoned that acute supplementation with high doses of RV would improve FMD and VO2 kinetics during low-intensity exercise in older CAD patients after rehab following coronary revascularization.
Methods
Study design and population
After ethical approval and informed consent, CAD patients from a cardiac rehabilitation program (n = 10; 9 male) participated in a placebo-controlled single-blind, crossover study comprising three visits to the laboratory within three weeks. Patients were included if they were between 45 and 75 years of age, had a history of CAD and had either a percutaneous coronary intervention (PCI), or post-coronary artery bypass graft (CABG). All patients were on optimal medical treatment and stable regarding symptoms and pharmacotherapy for at least six weeks. Exclusion criteria included: significant undercurrent illness in the last six weeks; known severe ventricular arrhythmia with functional significance; significant exercise-induced myocardial ischemia or arrhythmia, or any other disease that would limit exercise performance; the use of RV or antioxidant supplements within the three months prior to the study; and consumption of more than three drinks of alcohol per week. Smoking was allowed. Patients only participated upon their cardiologist’s approval. The approval criteria included the following: Participation in phase III cardiac rehabilitation program; Stable for at least 6 weeks with regards to symptoms and medication; having undergone PCI or CABG; No signs of significant ventricular arrythmia or ischemia during cardiopulmonary exercise testing; No co-morbidity that might represent a significant influence on 1-year prognosis (e.g. cancer). Medication taken by the participants included anti-hypertensive drugs (n = 9); beta-blockers (n = 6); anti-thrombotics (n = 9); lipid-lowering (n = 9) and glucose-lowering (n = 2) drugs.
On the first visit, patients performed a screening maximal cardiopulmonary exercise test on a cycle ergometer (Ergometrics 800 S. Ergometrics, Bitz, Germany). During the test, an initial workload of 20 W was increased by 20 W every minute until volitional exhaustion. A 12-lead electrocardiogram was recorded continuously as well as breath-by-breath gas exchange analysis (Oxycon Pro TM Jaeger, Carefusion 234, GMbH Hoechberg, Germany). Peak VO2 was defined as the highest 30-s average of VO2 at the end of the test.
Patients returned to the laboratory on two more occasions: once after supplementation with RV and once after supplementation with placebo. During each visit, patients underwent measurements of endothelial function in the brachial artery followed by assessment of VO2 kinetics. All tests were performed between 08:00 and 10:00 am in a fasted state, one hour after ingestion of the last capsule (RV or placebo), and a seven-day wash-out period in-between. All patients provided informed written consent, completed the crossover study and none experienced adverse effects. Population characteristics are described in .
Table 1. Characteristics of the participants.
Chemical compounds
Both RV (98.57% pure, polygonum cuspidatum extract; microcrystalline cellulose, 21st Century Alternatives, GB) and placebo were given in capsules of 330 mg each. The capsules were identical in appearance and weight and presented in white bottles. Participants were instructed to consume one capsule every 8 hours (i.e. three times per day) for three days and to ingest the last capsule one hour before the experimental session in the morning of the fourth day before each of the two visits. Participants were blinded to the supplementation.
Endothelial function
Brachial artery images were obtained with a 12-MHz high-resolution linear-array vascular ultrasound scanning transducer (Vivid 7; GE Healthcare). A BP cuff was placed proximal to the transducer on the forearm, and after 10 minutes of rest, the cuff was inflated to at least 200 mmHg, or 50 mmHg over the systolic pressure, for exactly five minutes. Longitudinal brachial artery images were recorded continuously 30 seconds before occlusion and for 150 seconds following cuff deflation. Diameter measurements and image analysis was conducted using edge-detection software (Flomedi, Brussels, Belgium) and FMD calculation were carried out as previously described [Citation11]. The within- and between-day variability has previously been determined in a cohort of patients with coronary artery disease as 10% and 11%, respectively [Citation11].
VO2 kinetics
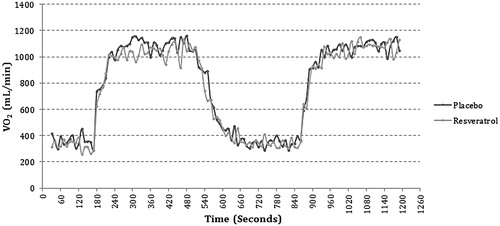
Following assessment of FMD, measurement of VO2 kinetics started with a three-minute sitting rest on the cycle ergometer to obtain resting VO2 data. Next, participants were instructed to cycle at a rate of 70 rpm against a resistance corresponding to 30% of peak load for six minutes, then to remain seated on the bike for additional six minutes, after which they performed a second six-minute bout. Exercise VO2 kinetics were calculated and expressed as mean response time (MRT).
Statistical analysis
Statistical analyses were performed using SPSS (Chicago, IL, USA). All data are expressed as mean ± SD, unless specified otherwise. The Shapiro–Wilk test was used to check normality of the data. A repeated-measures ANOVA with as within factor RV (placebo vs. RV) and between factor condition (PCI vs. CABG) was used to assess the effects of RV treatment, and PCI and CABG, respectively. To assess the effects of RV on VO2-kinetics parameters in PCI and CABG patients a Wilcoxon signed-ranked test was applied. Statistical significance was established at p < .05 (2-tailed).
Results
Endothelial function
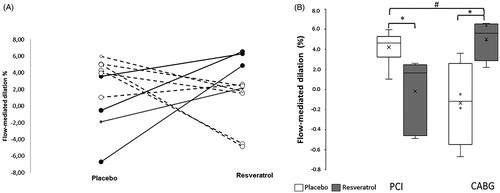
Supplementation with RV did not result in significant changes of baseline brachial artery diameters (; RV data not shown). There was a significant “revascularization method” * RV interaction (p = .004). This was reflected by an RV-induced reduction in FMD in the patients who underwent PCI, and an RV-induced increase in FMD in the patients who had a CABG ().
Figure 1. A: Individual and B: average resveratrol-induced changes in flow-mediated dilation (FMD) in patients that had a percutaneous intervention (PCI: —^) or post-coronary artery bypass graft (CABG: _____●). *: Significantly different from corresponding baseline; #: significantly different from other group. Data are mean ± SEM. Interaction resveratrol × intervention p = 0.004.
Oxygen kinetics
The VO2 responses during exercise before and after supplementation with RV are shown in and . The MRT, VO2 steady-state, and oxygen deficit did not differ significantly between PCI and CABG patients and in neither group did RV have any significant effect on MRT, the VO2 steady-state, or oxygen deficit.
Table 2. The effects of resveratrol on oxygen kinetics.
Discussion
The key finding of our study is that RV has differential effects on vascular function in older CAD patients, depending on the type of revascularization method that they have undergone. In the subset of patients who had undergone the PCI procedure, while their FMD values were within the normal expected range [Citation8], acute RV supplementation, led to a significant reduction in FMD. On the contrary, for the CABG patients, supplementation led to a significant improvement in FMD.
In humans, 1 g RV per day is considered the highest oral dose that does not elicit any deleterious side effects [Citation12]. The bioavailability of RV is low in humans due to rapid metabolism in the liver with a plasma half-life of 9.2 h [Citation13]. Even though absorption from orally consumed RV is at least 70%, a single 25-mg dose leads to peak plasma levels of RV metabolites of 2 µM and only trace amounts of non-metabolized RV [Citation13]. The dose used in our study, which was 10-fold higher than previous studies [Citation13] should have led to ∼80 µM of RV metabolites in plasma after the last meal of the day and to ∼26 µM just before evaluation of physiological parameters. Thus, the dose in our study should be more than adequate to study the effects of RV on FMD and VO2 kinetics in CAD patients.
RV is thought to act through SIRT1, a protein deacetylase involved in the transcriptional regulation of cell metabolism [Citation14]. Low SIRT1 activity has been suggested to play an important role in the development of atherosclerosis in human cardiac coronary vessels [Citation14]. In isolated vessels, RV improved endothelium-dependent and independent relaxation and aerobic capacity via activation of SIRT1, increased production of NO, and the activity of both eNOS and nNOS [Citation15]. In obese men, acute and chronic supplementation with RV reduced BP and pro-inflammatory cytokines [Citation16], and improved FMD [Citation17]. As older patients with CAD present high levels of pro-inflammatory cytokines, we hypothesized that a three-day supplementation with high doses of RV would improve FMD, oxygen supply and consequently, oxidative metabolism in the working muscle. Recent studies have reported no improvements in BP [Citation10] or FMD within a similar range upon RV treatment [Citation17], as well as no effect on aged mice skeletal muscle function [Citation18]. Part of this controversy might relate to the dose of RV, whereby low doses of RV in vitro (1–10 µM) have stimulated proliferation of human umbilical vein endothelial cells, and promoted myoblast sprouting and migration whereas high doses were detrimental [Citation19].
CAD patients can be stratified according to clinical presentation and treatment type, which is key in determining disease outcome [Citation20]. The two main revascularization methods include PCI and CABG. Here, we show an FMD component in patients who had undergone PCI, but no/minimal FMD in those who had CABG. Such a reduction in FMD after CABG has been reported before and was ascribed to reduced shear stress forces rather than a faulty endothelium [Citation21]. Others, however, also observed a decreased FMD in patients who had PCI, which was ascribed to excessive production of inflammatory cytokines, ET-1 and impaired cleavage of pro-BNP to BNP [Citation22,Citation23]. We did not have a healthy control group and it could well be that even though there was FMD after PCI, it would be less after the intervention.
It is interesting to note that the patients who underwent PCI showed no loss of endothelial function [Citation8]. However, compromised endothelial function was evident in the patients with CABG. This is not surprising, given the fact that CABG patients usually present with more severe CAD, affecting multiple vessels and having co-morbidities [Citation24]. Within our CABG cohort, 2 out of the 4 patients also had diabetes, and had double the triglyceride blood concentration levels, in comparison to the PCI patients (where only one of the 6 PCI patients had diabetes). Treatment with RV reduced FMD in the subset of patients with PCI but significantly improved it in CABG. To date, there is little work published on the effects of RV on endothelial dysfunction, but it has been reported in rabbits that RV treatment for 8 weeks preserves endothelial function by reducing the number of circulating endothelial cells after CABG [Citation25]. In addition, in isolated murine femoral arteries, we have shown that RV improves acetylcholine-induced dilation but not flow-mediated dilation, suggesting that RV might improve dilation via endothelial independent mechanisms, by acting directly on the smooth muscle cell layer [Citation26]. Combining these observations in isolated femoral arteries with our current observations leads to cautiously speculate that in CABG the endothelium may be damaged, a conclusion opposite to that posed by others [Citation21], while the endothelium is intact in PCI. Whatever the mechanism, our study points to new avenues of research and suggests that RV might have different effects on endothelial function depending on shear stress and/or inflammatory status [Citation5]. We believe this is important considering the broad range of putative positive effects, however poorly demonstrated, currently ascribed to RV. Consequently, RV may be an important adjunct therapy for CABG, but not PCI patients. Patient stratification and RV treatment according to the type of revascularization method may be important in managing prognosis of CAD patients.
VO2 kinetics are significantly correlated (r=–0.80) with peak VO2 [Citation27] and skeletal muscle oxidative capacity (0.33) [Citation28]. Previous studies found that RV improved exercise and aerobic capacity via increased mitochondrial function [Citation29] and prevented the obesity-induced insulin resistance in the skeletal muscle of mice [Citation30]. However, such benefits of RV have not been found in healthy, sedentary adults supplemented with ∼1 g of RV per day for one week [Citation31]. Similarly, we did not observe any improvement of VO2 kinetics following RV supplementation.
Limitations to our study include possible masking of effects of RV by the prescribed medication. For instance, some participants took anti-hypertensive and lipid-lowering agents that may already have a positive impact on the vasodilatory capacity of arteries. Further, medication for hypercholesterolaemia can influence cardiovascular parameters and skeletal muscle function [Citation32]. While the nutraceutical potential of RV in CAD patients may only be relevant if combined with commonly prescribed medication, RV may emerge as an alternative option for patients resistant or intolerant to standard therapy.
Conclusions
In conclusion, short-term treatment with high doses of RV seems to have differential effects on vascular function in elderly CAD patients, depending on the type of revascularization method experienced. While RV reduced FMD in patients who underwent PCI, it significantly improved it in patients after CABG. Our findings have important implications for personalized treatment and stratification of older CAD patients.
Ethical approval
Approval was granted by the medical ethical committee of UZ Leuven/KU Leuven (ML9734). The study followed the guidelines of the Declaration of Helsinki and Tokyo.
Informed consent
CAD patients from a cardiac rehabilitation program were asked to participate and all participants gave written informed consent.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Currie KD, McKelvie RS, Macdonald MJ. Brachial artery endothelial responses during early recovery from an exercise bout in patients with coronary artery disease. BioMed research international. 2014;2014:591918.
- Bruning RS, Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Progr Cardiovasc Dis. 2015 ;57:443–453.
- Buckley AF, Bossen EH. Skeletal muscle microvasculature in the diagnosis of neuromuscular disease. J Neuropathol Exp Neurol. 2013;72:906–918.
- Korzeniewski B, Zoladz JA. Biochemical background of the VO2 on-kinetics in skeletal muscles. J Physiol Sci. 2006 ;56:1–12.
- Diaz M, Degens H, Vanhees L, et al. The effects of resveratrol on aging vessels. Exp Gerontol. 2016;85:41–47.
- Tang Y, Xu J, Qu W, et al. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J Nutr Biochem. 2012;23:1410–1416.
- Li X, Dai Y, Yan S, et al. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clin Exp Hypertension (NY). 2016;38:287–293.
- Wong RH, Howe PR, Buckley JD, et al. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856.
- Scribbans TD, Ma JK, Edgett BA, et al. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl Physiol Nutr Metab. 2014 ;39:1305–1313.
- Gliemann L, Schmidt JF, Olesen J, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059.
- Onkelinx S, Cornelissen V, Goetschalckx K, et al. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79–84.
- Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLOS One. 2011;6:e19881.
- Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382.
- Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. JAT. 2010;17:970–979.
- Arrick DM, Sun H, Patel KP, et al. Chronic resveratrol treatment restores vascular responsiveness of cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart Circul Physiol. 2011;301:H696–703.
- Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622.
- Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertension. 2013;31:1819–1827.
- Ballak SB, Jaspers RT, Deldicque L, et al. Blunted hypertrophic response in old mouse muscle is associated with a lower satellite cell density and is not alleviated by resveratrol. Exp Gerontol. 2015;62:23–31.
- Bosutti A, Degens H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep. 2015;5:8093.
- Buccheri S, D’Arrigo P, Franchina G, et al. Risk stratification in patients with coronary artery disease: a practical walkthrough in the landscape of prognostic risk models. Interventional Cardiol (London). 2018;13:112–120.
- Dedichen HH, Hisdal J, Skogvoll E, et al. Reduced reactive hyperemia may explain impaired flow-mediated dilation after on-pump cardiac surgery. Physiol Rep. 2017;5:e13274.
- Han B, Ghanim D, Peleg A, et al. Loss of systemic endothelial function post-PCI. Acute Cardiac Care. 2008;10:79–87.
- Patti G, Pasceri V, Melfi R, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75.
- Sangalli F, Guazzi M, Senni S, et al. Assessing endothelial responsiveness after cardiopulmonary bypass: insights on different perfusion modalities. J Cardiothoracic Vascular Anesthesia. 2015;29:912–916.
- Zhu Y, Feng B, He S, et al. Resveratrol combined with total flavones of hawthorn alleviate the endothelial cells injury after coronary bypass graft surgery. Phytomedicine. 2018 ;40:20–26.
- Diaz M, Parikh V, Ismail S, et al. Differential effects of resveratrol on the dilator responses of femoral arteries, ex vivo. Nitric oxide. 2019;92:1–10.
- Powers SK, Dodd S, Beadle RE. Oxygen uptake kinetics in trained athletes differing in VO2max. Europ J Appl Physiol. 1985;54:306–308.
- Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol Ser A. 2013;68:447–455.
- Murase T, Haramizu S, Ota N, et al. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10:423–434.
- Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122.
- Voduc N, la Porte C, Tessier C, et al. Effect of resveratrol on exercise capacity: a randomized placebo-controlled crossover pilot study. Appl Physiol Nutr Metab. 2014;39:1183–1187.
- Ceriello A, Assaloni R, Da Ros R, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518–2524.