Abstract
Objectives. Coronary revascularisation and intra-aortic balloon pump (IABP) has been considered the gold standard treatment of acute coronary syndrome with cardiogenic shock, recently challenged by the SHOCK II study. The aim of this non-randomised study was to investigate the long term prognosis after immediate IABP supported angiography, in patients with acute chest pain and cardiogenic shock, treated with percutaneous coronary intervention (PCI), cardiac surgery or optimal medical treatment. We assessed data from 281 consecutive patients admitted to our department from 2004 to 2010. Results. Mean (±SD) age was 63.8 ± 11.5 (range 30–84) years with a follow-up of 5.6 ± 4.4 (0–12.7) years. Acute myocardial infarction was the primary diagnosis in 93% of the patients, 4% presented with unstable angina pectoris and 3% cardiomyopathy or arrhythmias of non-ischemic aetiology. Systolic blood pressure at admittance was 85 ± 18 mmHg and diastolic 55 ± 18 mmHg. Thirty day, one- and five-year survival was 71.2%, 67.3% and 57.7%, respectively. PCI was performed immediately in 70%, surgery was done in 17%, and 13% were not eligible for any revascularisation. Independent variables predicting mortality were medical treatment vs revascularisation, out-of-hospital cardiac arrest, and advanced age. Three serious non-fatal complications occurred due to IABP treatment, i.e. 0.001 per treatment day. Conclusions. We report the use of IABP in patients with acute chest pain admitted for angiography. Long-term survival is acceptable and discriminating factors were no revascularisation, out-of-hospital cardiac arrest and age. IABP was safe and feasible and the complication rate was low.
Introduction
Cardiogenic shock is a life-threatening state of congestion, hypoperfusion and tissue hypoxia due to reduced cardiac output. Acute myocardial infarction with left ventricular dysfunction or mechanical complications is the most frequent cause of cardiogenic shock, seen in 4–8% of patients (Citation1–3). Consequently, coronary revascularisation is the most important and only validated therapeutic intervention to improve survival (Citation2,Citation4–7). However, despite early coronary revascularisation and improved medical treatment, in-hospital mortality in cardiogenic shock remains high, approximately 50% (Citation8,Citation9).
Since 1968, the intra-aortic balloon pump (IABP) has been the most widely used haemodynamic support in patients with cardiogenic shock (Citation10). The IABP increases diastolic blood pressure, increases coronary perfusion, reduces left ventricular afterload, and decreases myocardial workload and oxygen consumption. Until recently, percutaneous coronary intervention (PCI) and IABP has been considered the gold standard treatment of acute myocardial infarction with cardiogenic shock. Due to the neutral results from the SHOCK II study, i.e. IABP versus medical treatment in patients with ST-elevation myocardial infarction (STEMI) and cardiogenic shock, the routine use of IABP has been a subject of debate (Citation11,Citation12). SHOCK II was a warranted and well conducted randomised controlled trial with major impact on the treatment of cardiogenic shock and the routine use of IABP. Except for mechanical complications in acute myocardial infarction (e.g. severe mitral regurgitation, ventricular septum rupture) or large anterior infarctions the recommendation of routine use of IABP has now been downgraded in guidelines from the European Society of Cardiology, American Heart Association, and American College of Cardiology ESC/AHA/ACC (Citation13–17). Due to lack of randomised controlled trials, recommendations and level of evidence have always been weak for inotropes, vasopressors, vasodilators, circulatory support with percutaneous left ventricular assist devices (e.g. Impella®) or extracorporeal membrane oxygenation (ECMO). Thus, reperfusion remains the only validated therapy in cardiogenic shock and must be performed rapidly. The use of inotropic support and cardiac assist devices are most often decided by heart teams based on pre-defined algorithms adapted to individual clinical situations.
Data regarding long-term prognosis in non-selected patients with acute chest pain and cardiogenic shock is scarce. In the present retrospective single centre study, we report IABP supported immediate angiography, treatment, survival and prognostic risk factors from a large cohort of such patients with a very long-term follow-up.
Material and methods
The present study is retrospective by chart review. Data was obtained from our database of all IABP treated patients, approved by the institutional data protection board, Oslo University Hospital, Rikshospitalet. The study was performed according to the Helsinki declaration and did not require ethical approval due to its nature of being a quality control study based on a hospital registry. All authors are employees at Oslo University Hospital and have no potential conflict of interest, i.e. no funding or grants.
From 1 January 2004 to 31 December 2009, 281 patients were admitted to our hospital with refractory chest pain and refractory cardiogenic shock. The patients were immediately transferred to the catheterisation laboratory for IABP supported standard coronary angiography. The IABP was inserted using a sheatless Seldinger technique via a femoral artery. IABP was inserted before or immediately after angiography/PCI depending on the scenario and clinical development. DatascopeTM. IABP balloon catheters (Size 7.5/8 Fr 30/40/50 cc) and a DatascopeTM console were used. Duration of treatment days was recorded from insertion to removal of IABP after stabilisation, surgery or death. The right radial artery was used for coronary angiography and subsequently for PCI if suitable for treatment, and a non-ionic contrast medium was used. The coronary angiograms were reviewed to the point of consensus by a heart team on call before the revascularisation strategy was decided in each patient. Based on the coronary angiography and clinical evaluation, the patients were non-randomly selected for three different treatment strategies: PCI (n = 196), cardiac surgery (n = 48), or medical treatment as the best possible option (n = 37). The invasive procedure, intensive care- and surgical treatment followed commonly accepted routines.
Statistical analysis was performed using IBM SPSS for Windows version 23 (Armonk, NY, USA). Survival rates were calculated by the Kaplan–Meier method and compared by the log-rank test. Multiple regression was calculated from univariate variables with p < .05. For all comparisons, p < .05 was considered significant. Data are presented as mean ± standard deviation (SD) if not otherwise stated. Closing date for survival data was 01.12.2018.
Results
Baseline characteristics for the cohort of 281 patients are presented in . Age was 63.8 ± 11.5 (range 30–84) years, male/female: 203 (72%)/78 (28%), with 96 (34%) of the patients above 70 and 16 (5.7%) over 80 years of age, respectively. Follow-up was 5.6 ± 4.4 (0–12.7) years. AMI was the primary diagnosis in 93% of the patients, 4% unstable angina pectoris and 3% cardiomyopathy or arrhythmias of non-ischemic aetiology.
Table 1. Baseline characteristics, diagnostic variables, and treatment of patients with IABP supported immediate angiography in acute chest pain and cardiogenic shock.
Blood pressure was registered in 270 (96%) of the patients at admission with a systolic value of 85 ± 18 mmHg and diastolic blood pressure of 55 ± 18 mmHg. In three patients, blood pressure was not documented, whereas the registration was regarded as unreliable in another eight patients. Swan-Ganz catheter data and blood gas analysis are not available. Coronary angiography revealed significant obstructive coronary artery disease in 273 (97%). Left main stem, left anterior descending artery, ramus circumflexus, and right coronary artery were significantly stenotic or occluded in 37/8, 97/122, 69/60, 73/93 lesions, respectively ().
In the PCI group [n = 196 (70%)], 288 coronary arteries were treated, i.e. 1.3 vessels per patient. In surgically treated patients [n = 48 (17%)], interventions were 27 coronary artery bypass grafting (CABG), 14 mitral valve repairs/replacements, 13 ventricular septal ruptures repairs, two heart transplants and two left ventricular assist devices (LVADs). The majority of the combined surgical interventions were CABG with ventricular septal ruptures repair and/or mitral valve surgery. In those not suitable for any revascularisation [n = 37 (13%)], optimal medical treatment was the only treatment option. Data on use of inotropes, pressors, vasodilators, and renal replacement therapy is not available.
Duration of IABP treatment was 5.2 ± 9.8 (range 0–89) days. In three patients serious vascular non-fatal complications were noted (i.e. two peripheral embolisms in the lower extremities and one in the intestine, respectively), i.e. 0.001 per treatment day.
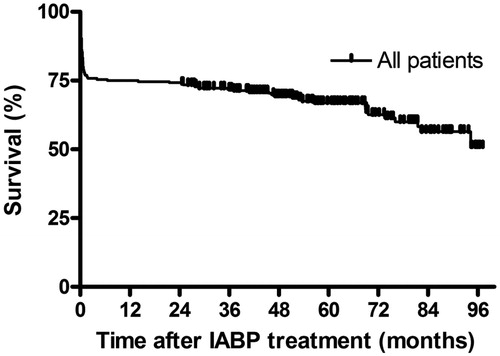
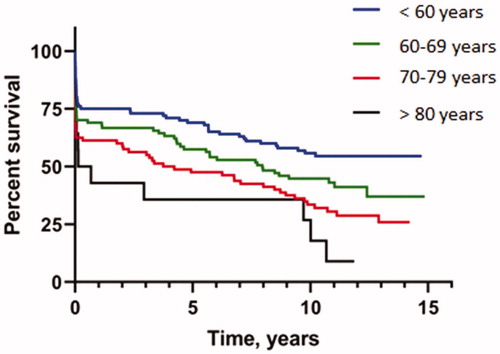
shows the survival of the total cohort. Thirty day, six months, one and five year survival was 71.2%, 68.4%, 67.3% and 57.7%, respectively. Survival for the total cohort was 6.8 ± 0.3 years, decreasing with increasing age (). For those over 80 years of age (n = 14), 30-days mortality was 64%, and median survival was 0.13 (0.01–0.26) years.
Figure 2. Subgroup analysis (Kaplan–Meier time-to-event curve) for survival in different age groups.
Independent variables predicting mortality were medical treatment vs revascularisation (OR 2.51, CI 1.02–6.12, p = .046), out-of-hospital cardiac arrest (3.03, 1.6–5.8, p = .001), and age as continuous variable (1.06, 95% 1.04–1.09, p < .001). Gender (p = .81), left main stem or left anterior descending artery as culprit lesion (0.075), smoking (p = .17) and diabetes (p = .095) did not influence long-term survival.
The non-randomised and selective design of the cohort prohibits any comparison between the different treatment groups. Patients scheduled for surgery had less previously diagnosed cardiovascular disease (p = .046), use of ventilator (p < .005), and out-of-hospital cardiac arrest (p < .005). One-vessel disease had better survival than multi-vessel disease (p < .0001). Treatment of the culprit lesion only in multi-vessel disease demonstrated similar survival compared to full revascularisation (p = .506). Patients not suitable for PCI or surgery had the highest mortality with survival 5.4 ± 0.8 vs 7.1 ± 0.4 years (p = .032).
Discussion
In acute myocardial infarction complicated by cardiogenic shock, early revascularisation is the single most important therapeutic intervention to improve survival, and has a class IA recommendation in scientific guidelines (Citation15). After the SHOCK I study, revascularisation with PCI and mechanical circulatory support with IABP was widely used in the treatment of acute myocardial infarction with cardiogenic shock. However, in the SHOCK II study, IABP and optimal medical treatment demonstrated similar survival in patients with acute myocardial infarction and cardiogenic shock (Citation9). Eventually, short-term IABP was downgraded to a class IIb C recommendation in STEMI with cardiogenic shock, and routine use of IABP was not recommended (class III B) (Citation11,Citation12,Citation15,Citation17). Interestingly, inotropes and vasopressors enhancing vascular tone and cardiac output have also failed to demonstrate treatment effect (i.e., reduction of mortality) in cardiogenic shock, and therefore also have a class IIb C recommendation (Citation18). Critics have been raised regarding lower event rates in the control group than expected, the amount of crossovers, and high percentage of patients with cardiac arrest in need of post resuscitation care (e.g. temperature control, assisted ventilation) in the SHOCK II study. Moreover, inclusion of patients was possible up to 12 hours after symptom onset. Late revascularisation with non-viable myocardium and non-present ischemia does not promote the effect of IABP on the coronary circulation. In the CRISP AMI trial, PCI and IABP in cardiogenic shock demonstrated a survival benefit for the larger anterior ST elevations with resolution of the ST segment after PCI supporting the use of IABP in acute ongoing ischemia (Citation19). In a non-viable myocardium, use of IABP will lead to improved hemodynamic variables, but no salvation of the myocardium.
Independent of the criticism raised, the results from SHOCK II study have added valuable information to a very challenging patient population and therefore gained impact on treatment algorithms and guidelines. Thus, the currently accepted indications for the use of IAPB are limited to mechanical complications in acute myocardial infarction such as acute mitral regurgitation, particularly due to papillary muscle rupture, or ventricular septal rupture. Moreover, left ventricular unloading in ECMO treated patients, bridging unstable patients before and after cardiac surgery, adjunctive therapy in high risk or complicated PCI, and bridging to further therapy in refractory heart failure or ventricular arrhythmias are also commonly accepted indications despite lack of evidence from randomised trials.
The majority of published cardiogenic shock studies are based on registries whereas only a few are randomised controlled trials (Citation8,Citation20). Thus, comparing the results of the present study with those of previous trials is not straightforward. However, our results are comparable with those of other retrospective registries regarding mortality up to one year. Long-term survival (five years) in our study was 57.7% which is acceptable compared to earlier studies (Citation21). Our single arm study does not challenge trials with control groups, but nevertheless indicates that long-term survival was good in a historical selected cohort. Moreover, the complication rate was low in an experienced high-volume center.
Management of cardiogenic shock is challenging. Cardiogenic shock with ongoing ischemia has to be recognised early with only a few clinical variables available, and at the same time, decision making and treatment has to be rapid. Thus, immediate revascularisation is the primary objective, but non-invasive measurement (echocardiography) and invasive monitoring (arterial and central venous line, pulmonary artery catheter) is also crucial. However, proper invasive evaluation is difficult to obtain as time to open artery is the most important variable. Thus, clinical skills and easily available variables therefore remain the cornerstone of the diagnosis of cardiogenic shock.
Lately, more advanced and effective mechanical circulatory support like Impella® and ECMO is more frequently used. However, the results were also neutral in a recent randomised controlled trial comparing IABP with Impella® in patients with STEMI and cardiogenic shock (Citation20). This was a surprise, taking into account that an Impella® adds up to 4 liters/minute to the cardiac output, whereas a maximum of 0.5 is possible for IABP. In a recent retrospective analysis of patients with acute myocardial infarction with cardiogenic shock, the use of an Impella® was not associated with lower 30-day mortality compared with propensity matched patients from the SHOCK II trial, but with higher rates of bleeding (Citation22).
In some institutions, the even more powerful ECMO, which also oxygenates the blood in addition to being circulatory support, is widely used. The scientific evidence for this use of ECMO is absent with no randomised controlled trials available, most probably because they are challenging to perform. Logically, Impella® should be the preferred mechanical circulatory support since it unloads the left ventricle with antegrad flow-direction, but unfortunately does not promote pulsatility and reverse remodelling (Citation21). In contrast, an ECMO increases afterload considerably with its retrograd flow-direction and is dependent on myocardial contractile reserves to achieve survival without bail-out with a permanent left ventricular assist devices or heart transplant (Citation23). Thus, guidelines emphasize that mechanical circulatory support should be preferred in the most serious and refractory cardiogenic shock, but no preference to insertion time or type of device is outlined (Citation24). IABP is still widely used in patients with circulatory insufficiency despite the downgraded recommendation in guidelines (Citation25), and is still a treatment option in our department. IABP carries the favour of possible long-term therapy compared to Impella® and ECMO, and further research for portable and home based counterpulsation heart assist is reported and under development (Citation26).
Full revascularisation in single vessel disease had the best survival rates in our cohort, and treatment of the culprit lesion only in multi-vessel disease was non-significantly better than full revascularisation. This is in accordance with the recent CULPRIT SHOCK trial, where treating the culprit lesion only was superior to full revascularisation in multi-vessel disease regarding mortality and need of renal replacement therapy after 30 days (Citation27). After one year, however, the difference between the two treatment strategies was not significant, but with higher rates of readmissions due to need for revascularisation in the culprit only group.
Revascularisation is also favourable and recommended in the elderly, but historically patients aged 75 years or more with cardiogenic shock have been treated less aggressive both in the catheterisation laboratory and in the intensive care unit (Citation28). In cardiogenic shock, age is a predictor for mortality, but the cut-off for use of IABP is not set. In our cohort age > 80 years experienced an increased early mortality, whereas survival beyond one year was almost similar to the younger groups. This may be due to a small and selective sample size, but a 30-day survival of 36% indicate that decision making has to be individually tailored, considering life expectancy, comorbidity, bleeding risk, cognitive and functional status, and patient preference.
The present study has several limitations. First, the retrospective design of the study with no control group may cause a selection bias which could explain the favorable results. Second, the results were obtained in a single experienced center and may not necessarily be reproducibly in other centers. Third, a small number of patients limits comparison of subgroups. Fourth, we cannot rule out the possibility of underreporting of adverse events due to the retrospective design of the study.
Conclusion
Coronary revascularisation is the most important and only validated therapeutic intervention to improve survival in cardiogenic shock. We have reported the use of immediate IABP in patients admitted with acute chest pain and cardiogenic shock for acute angiography. Long-term survival is acceptable and discriminating factors are age, no revascularisation and out-of-hospital cardiac arrest. Full revascularisation in one-vessel disease with PCI had the best survival. Gender, left main stem or left anterior descending artery as culprit lesion, smoking and diabetes did not affect survival. IABP is safe and feasible and complications are few.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Goldberg RJ, Gore JM, Alpert JS, et al. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N Engl J Med. 1991;325:1117–1122.
- Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–1219.
- Kolte D, Khera S, Dabhadkar KC, et al. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non-ST-elevation myocardial infarction. Am J Cardiol. 2016;117:1–9.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511–2515.
- Lee L, Erbel R, Brown TM, et al. Multicenter registry of angioplasty therapy of cardiogenic shock: initial and long-term survival. J Am Coll Cardiol. 1991;17:599–603.
- Bangalore S, Gupta N, Guo Y, et al. Outcomes with invasive vs conservative management of cardiogenic shock complicating acute myocardial infarction. Am J Med. 2015;128:601–608.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634.
- Thiele H, Ohman EM, Desch S, et al. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–1230.
- Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38:3523–3531.
- Kantrowitz A, Tjonneland S, Freed PS, et al. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:113–118.
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296.
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–1645.
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425.
- van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the american heart association. Circulation. 2017;136:e232–e268.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161.
- Tariq S, Aronow WS. Use of inotropic agents in treatment of systolic heart failure. Int J Mol Sci. 2015;16:29060–29068.
- Kohl LP, Leimberger JD, Chiswell K, et al. Clinical characteristics and outcomes after unplanned intraaortic balloon counterpulsation in the Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction trial. Am Heart J. 2016;174:7–13.
- Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69:278–287.
- Schrage B, Ibrahim K, Loehn T, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139:1249–1258.
- Katz SD, Smilowitz NR, Hochman JS. Another nail in the coffin for intra-aortic balloon counterpulsion in acute myocardial infarction with cardiogenic shock. Circulation. 2019;139:404–406.
- Chung BB, Sayer G, Uriel N. Mechanical circulatory support devices: methods to optimize hemodynamics during use. Expert Rev Med Devices. 2017;14:343–353.
- Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–1535.
- Romeo F, Acconcia MC, Sergi D, et al. Percutaneous assist devices in acute myocardial infarction with cardiogenic shock: review, meta-analysis. World J Cardiol. 2016;8:98–111.
- Jeevanandam V, Song T, Onsager D, et al. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J Heart Lung Transplant. 2018;37:1–6.
- Thiele H, Akin I, Sandri M, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379:1699–1710.
- Tegn N, Abdelnoor M, Aaberge L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (after eighty study): an open-label randomised controlled trial. Lancet. 2016;387:1057–1065.