 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives. Heart failure (HF) impairs resting myocardial energetics, myocardial mitochondrial performance, and maximal oxygen uptake (VO2max). Exercise training is included in most rehabilitation programs and benefits HF patients. However, the effect of exercise intensity on cardiac mitochondrial respiration and concentrations of the key bioenergetic metabolites phosphocreatine (PCr), adenosine triphosphate (ATP), and inorganic phosphate (Pi) is unclear. This study aimed to investigate the effects of exercise training at different intensities in rats with HF. Methods. Rats underwent myocardial infarction or sham operations and were divided into three subgroups: sedentary, moderate intensity, or high intensity. The impact of HF and 6 weeks of exercise training on energy metabolism was evaluated by 31P magnetic resonance spectroscopy and mitochondrial respirometry. The concentrations of PCr, ATP, and Pi were quantified by magnetic resonance spectroscopy. VO2max was measured by treadmill respirometry. Results. Exercise training increased VO2max in sham and HF. PCr/ATP ratio was reduced in HF (p < .01) and remained unchanged by exercise training. PCr concentration was significantly lower in HF compared to sham (p < .01). Moderate and high-intensity exercise training increased ATP in HF and sham. HF impaired complex I (CI) and complex II (p = .034) respiration. High-intensity exercise training recovered CI respiration in HF rats compared to HF sedentary (p = .014). Conclusions. Exercise training improved cardiac performance, as indicated by increased VO2max and higher exercise capacity, without changing the myocardial PCr/ATP ratio. These observations suggest that the PCr/ATP biomarker is not suited to evaluate the beneficial effects of exercise training in the heart. The exact mechanisms require further investigations, as exercise training did increase ATP levels and CI respiration.
Introduction
Coronary artery disease leading to myocardial infarction (MI) is a common cause of chronic heart failure (HF) with reduced ejection fraction, a pathophysiological condition in which the heart suffers from a long-term inability to pump blood at an adequate rate to meet the normal circulatory demand [Citation1]. Community-based studies show that about 30% of patients die from HF within 1 year after receiving the diagnosis [Citation2,Citation3]. Despite the significant improvement in prognosis with current therapies, longevity and quality of life are markedly reduced [Citation4].
Several lines of evidence suggest that exercise training benefits HF patients both physiologically and psychologically [Citation5,Citation6]. Exercise training can improve myocardium oxidative metabolism, ventricular function, and coronary circulation [Citation7–9]. Remaining challenges include understanding the underlying biology of exercise-related improvements in cardiac performance, including identification of the optimal intensity, frequency, and duration of exercise training for HF patients, and accurate quantification of the associated physiological alterations. Cellular maintenance of high-energy phosphate metabolism seems to be necessary to maintain myocardial function. Many studies suggest that the MI pathogenesis is associated with impaired bioenergetic metabolism and mitochondrial dysfunction [Citation10–13]. Although the molecular mechanisms are not fully understood, it seems that high-energy phosphates are downregulated as a result of abnormal mitochondrial function. The decrease in myocardial phosphocreatine (PCr)/adenosine triphosphate (ATP) ratio in HF patients correlates with the New York Heart Association functional classes, and is a predictor of cardiovascular mortality [Citation14,Citation15]. This finding has raised the possibility that measurements of phosphorous metabolites could be utilized in the evaluation of HF pathogenesis and response to treatment.
Magnetic resonance spectroscopy (MRS) is an important research approach in metabolomics. In chronic HF, phosphorus (31P) MRS can measure PCr and ATP [Citation16,Citation17], the critical metabolites involved in oxidative phosphorylation. Combined measures of 31P MRS and in situ mitochondrial respirometry may reflect the performance of HF myocardium and provide a further understanding of how exercise training remodels the HF energy metabolism. In this study, we aimed to determine the impact of different exercise training intensities on cardiac energy metabolism and mitochondrial respiration in HF. We acquired ex vivo 31P MRS, whole body maximal oxygen uptake (VO2max), and in situ mitochondrial respiratory measures from HF rats. We hypothesized that the impaired energy metabolism observed in chronic HF, would be restored after exercise training, and that energy metabolism would increase in an intensity-dependent manner.
Materials and methods
Heart failure model in rats
The present investigation was a substudy under a larger work by Stølen and Høydal et al. [Citation18], in which heart tissue from 15 HF rats (sedentary, moderate intensity trained, and high-intensity trained, n = 5 in each group) were randomly chosen from the original study. In addition, tissue from 15 sham-operated rats (divided in a sedentary, moderate-intensity trained, and high-intensity trained groups) were selected for MRS and metabolic measurements. The rats developed HF after permanent ligation of the descending coronary artery, as previously described [Citation19,Citation20]. In brief, female Sprague Dawley rats (280 grams from Taconic, Denmark) were randomized to sham operation or MI operation. Rats were anesthetized with 5% isoflurane, intubated, and ventilated with 1.5% isoflurane in a 70% O2/30% N2O mixture. The pericardium was opened after a left thoracotomy was performed, and in the MI group, the descending artery was ligated with a polyester suture (Ethibond 6-0, needle Rb-2, Ethicon; Norderstedt, Germany). The sham group underwent the same surgical process except for ligation of the descending coronary artery. Buprenorfin (0.04 mg/kg) was injected subcutaneously during the surgery and repeated 8 h thereafter to relieve pain. After four weeks, rats with a permanently ligated coronary artery were examined by echocardiography to determine the extent of MI. Only rats with an MI size of <40% of the left ventricle were included in further studies.
All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experiments were designed according to the guidelines from the Federation of European Laboratory Animal Science Associations (FELASA), EU animal research directive (86/609/EEC), Council of Europe (ETS 123), the EU directive (2010/63/EU), and approved by the national animal ethics committee. The 3 R’s (Replacement, Reduction, and Refinement) have specifically been addressed when designing the study.
Exercise training and VO2max testing
Four weeks after MI or sham surgery, VO2max was measured on an incline treadmill (25°) in a metabolic chamber as previously described [Citation21]. After the test, sham and MI rats were randomized to six subgroups (five animals in each subgroup): HF rats exercising at high-intensity (HF-high), moderate-intensity (HF-mod), and sedentary (HF-sed); sham rats at high-intensity exercise (Sham-high), Sham-mod, and Sham-sed. Both moderate and high-intensity exercise training started with 10 min warm-up at 50–60% VO2max. The 60 min high-intensity exercise included 10 cycles of 4 min running at 85–90% of VO2max and 2 min at 50% of VO2max. For moderate-intensity exercise training, rats ran of 4 min at 60–70% VO2max separated with 2 min at 50% VO2max for the same distance as their respective high-intensity group. Moderate-intensity groups exercised for about 80 min with gradually increased training duration to approximately 110 min at the end of the training period to match the high-intensity group with regard to distance. At the start and every 2 weeks, VO2max was measured in order to ascertain and adjust band speed of the treadmill to obtain the target exercise intensity. The exercise training protocols applied the overload principle by increasing band speed every 2 weeks according to those obtained during the VO2max test, so that the load remained at the same respective percentages of VO2max as the aerobic fitness increased in both training groups (moderate: 60–70%, high 85–90%) throughout the study. The protocols aimed at being isocaloric by adjusting the distance run by the moderate-intensity group to the same length as that run by the high intensity group). The sedentary rats did not exercise, and VO2max was measured before and after the training period. Rats with HF had significantly lower VO2max (p < .05, Supplementary Figure S1(A,B)). Exercise training significantly increased VO2max in both sham and HF rats, with the largest improvements at high intensity [Citation18].
Echocardiography
Echocardiography was performed during anaesthesia with 1.5% isoflurane in a 40% O2 60% N2O anesthesia mixture using a Vivid 7 scanner (GE Vingmed Ultrasound, Horten, Norway) with a phased-array matrix transducer. Ejection fraction and LV volumes were determined using a biplane (apical 4 and 2-chamber) modified Simpsons method [Citation22]. Fractional shortening was calculated by LV end diastolic dimension (LVEDD), and LV end systolic dimension (LVESD) as:
FS and EF were both significantly lower in the MI-operated rats (P < 0.01) and confirmed the presence of HF (Supplementary Figure S2(A,B)). High-intensity exercise training improved FS (p < .05) and EF (p < .01) in HF rats (Supplementary Figure S2(A,B)) [Citation18].
Tissue extraction
One day after the final exercise training, the rats were sacrificed for tissue extraction. During anesthesia, the hearts were quickly removed and placed in ice-cold saline for dissection. To avoid fibrotic and ischemic parts, tissue from the septum towards the apex was cut out from every heart and immediately frozen. Time from the removal of the heart to snap freeze was approximately 1 min and did not differ between groups. Extracts from frozen myocardial tissue samples were obtained for MRS experiments by using perchloric acid as previously described [Citation23].
In vitro MRS study
In vitro MRS studies were performed on a 14.1 T Bruker spectrometer (Bruker Avance III 600 MHz/54 mm US-Plus) equipped with a multinuclear QCI CryoProbe (Bruker BioSpin, Ettlingen, Germany). High-resolution 31P MR spectra were obtained with proton decoupling, a 30° flip angle, 8192 scans, repetition time of 3.62 s, and spectral width of 14,577 Hz into 47104 data points. MR spectra were analyzed with jMRUI software and metabolite signals were fitted by the AMARES method [Citation24]. Relative peak area of PCr and ATP were measured by normalizing the area under the individual peaks to a reference generated by adding up the area under curves of three metabolites: inorganic phosphate (Pi), ATP, and PCr. ATP was calculated as the sum of all three ATP peaks (ATPy+ATPα+ATPβ).
In situ mitochondrial respiration
Mitochondrial respiration was studied in permeabilized fibers using Oxygraph-2k (Oroboros Instruments, Austria) [Citation25,Citation26]. This method was used to determine how HF and exercise training affect maximal mitochondrial respiration of the cardiac muscle tissue. Small cardiac tissue samples (5–10 mg) from septum were put in a cryopreserving solution consisting of BIOPS (2.77 mM CaK2EGTA buffer, 7.23 mM K2EGTA buffer, 20 mM imidazole, 20 mM taurine, 50 mM 2-(N-morpholino) ethanesulfonic acid hydrate, 0.5 mM dithiothreitol, 6.56 mM MgCl2·6H2O, 5.77 mM Na2ATP and 15 mM Na2Phosphocreatine, pH 7.1) supplied with 30% DMSO and 10 mg/mL BSA and snap frozen as previously described [Citation26]. First, samples were thawn at room temperature for 15 min, then blotted with gauze to remove any residual DMSO from the tissue, and immediately transferred to a Petri dish containing 2 mL of BIOPS on ice. Using a microscope, heart muscle samples were carefully dissected using ice-cooled forceps until the single fibers were uniformly separated. Fibers were then permeabilized for 30 min in a solution containing 2 mL BIOPS and 20 µL of saponin stock solution (5 mg mL−1) resulting in a 50 µg mL−1 final saponin concentration. Next, fiber bundles were transferred to 2 mL ice-cold mitochondrial respiration medium (MiR05; 0.5 mM EGTA, 3 mM MgCl2·6H2O, 60 mM lactobionic acid, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM D-sucrose, 1 gL−1 BSA essentially fatty acid free, pH 7.1) and gently agitated for 10 min at 4 °C. After rinsing, samples were blotted on three layers of lens paper and then weighed (0.6–1.2 mg) before putting them in the high-resolution respirometer.
Complex I (CI) LEAK was assessed by addition of malate (2 mM) and glutamate (10 mM). Oxidative phosphorylation (OXPHOS) capacity was stimulated with saturating adenosine diphosphate (ADP) 5 mM. Succinate (10 mM) providing electrons for complex II (CII) was then added to reconstitute tricarboxylic acid cycle function with convergent electron flow through CI + CII. Cytochrome c (10 μM) was added to test for outer mitochondrial membrane integrity. No sample exceeded the cut-off of 10% increase in respiration and therefore none was excluded due to low membrane integrity after the addition of cytochrome c. To determine the capacity of the electron transfer system (ETS), the uncoupler carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, C10H5F3N4O (FCCP) was titrated stepwise (0.5 μM steps). Finally, rotenone (0.5 μM) was added to measure uncoupled CII respiration. All experiments were performed at 37 °C; values are expressed as pmol O2·s−1·mg−1 wet weight.
In contrast to the VO2max and MRS analysis (six subgroups), mitochondrial data were collected in four groups: HF-high, HF-mod, HF-sed, and sham operation (Sham). Rats in the sham group did not perform any exercise training.
Statistics
IBM SPSS Statistics 22 (Version 22.0, IBM Corp., Armonk, NY) and GraphPad Prism software (Version 7.0, Graphpad Software Inc. La Jolla, CA) were used for statistical analysis. Two-way analysis of variance (ANOVA), compared changes in VO2max and energy metabolism, using type of surgery (Sham, HF) and exercise training (sed, mod, and high) as factors. Non-parametric Mann–Whitney test after Kruskal–Wallis test was performed to compare the changes in mitochondrial respiration. All data are described as mean ± SD. p-value < .05 was considered statistically significant.
Results
In vitro MRS study
The key bioenergetic metabolites PCr, Pi, and the three peaks of ATP were detected with a high spectral resolution (with a mean full width at half maximum of 15 Hz for the PCr peak). Representative 31P MR spectra acquired from heart tissue extracts of the rat models are shown in .
Figure 1. Examples of high-resolution 31P MR spectra of heart muscle tissue extracts obtained from rats with heart failure (HF) and sham-operated controls with different exercise training programs: sedentary, moderate, and high intensity. Spectra are shifted to the right (except the Sham-Sed spectrum) for better visualization.
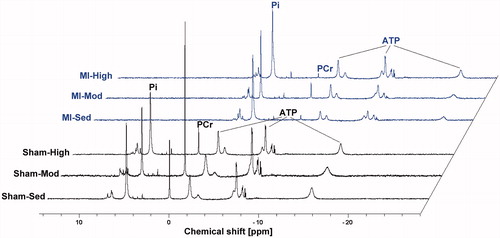
Within the sham groups, PCr/ATP ratio was lower after high-intensity training compared with moderate-intensity (p = .02), while this was not found in HF rats. HF rats had a significantly lower PCr/ATP ratio (p < .01) compared with the sham group. Exercise training did not change the PCr/ATP in HF rats ().
Figure 2. Myocardial phosphocreatine to adenosine triphosphate (PCr/ATP) ratio (A), ATP (B), and PCr (C) levels from rats with heart failure (HF) and sham-operated controls with different exercise training intensities. * denotes p-value < .05, and ** denotes p-value < .01.
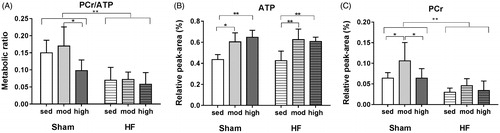
HF did not change the ATP level, and there was no significant difference between ATP peak-area measured in HF-sed and Sham-sed (). Moderate and high intensity exercise training caused an elevation in ATP concentration in both sham and HF (). ATP level increased significantly in the myocardium of HF rats with moderate-intensity exercise (47%, p < .01) and high-intensity exercise (42%, p < .01) compared with the HF-sed group (). A similar trend was observed in the sham groups, in which moderate and high-intensity exercise increased ATP levels by 40% (p < .01) and 51% (p < .01) respectively, compared to the sedentary group ().
Regardless of the exercise training, the relative level of PCr in HF was two-fold lower (p < .01) compared to the sham group. Moderate-intensity exercise training increased PCr level in the sham group, while exercise training did not change PCr level in HF ().
Mitochondrial function
Heart failure significantly impaired complex I respiration (, 9.62 ± 1.63 pmol s−1 · mg−1 in HF-sed group, compared with 16.20 ± 1.35 pmol s−1 · mg−1 in sham group, p = .03) and complex II respiration (, 140.00 ± 17.33 pmol s−1 · mg−1 in HF-sed group, compared with 223.19 ± 38.52 pmol s−1 · mg−1 in sham group, p = .03). Complex I respiration increased significantly after high-intensity exercise training in HF (28.46 ± 7.43 pmol s−1 · mg−1) compared with the HF-sed (9.62 ± 1.63 pmol s−1 · mg−1, p = .01) and sham (16.20 ± 1.35 pmol s−1 · mg−1, p = .03). However, exercise training did not improve complex II respiration.
Figure 3. Respiration rates of mitochondrial components described by oxygen flux (pmol·s−1·mg−1). (A) Oxygen consumption during oxidative phosphorylation (OXPHOS) at Complex I. (B) Oxygen consumption at Complex II, during the process of electron transfer system (ETS). (C) Oxygen consumption at both Complex I and Complex II. (D) Ratio of OXPHOS over ETS (P/E) when measuring the sum of oxygen consumption at both Complex I and Complex II. (E) OXPHOS efficiency calculated as the ratio of (P–L)/P, where P indicates the OXPHOS in OXPHOS stage and L indicates LEAK oxygen consumption. * denotes p-value <.05, and ** denotes p-value <.01.
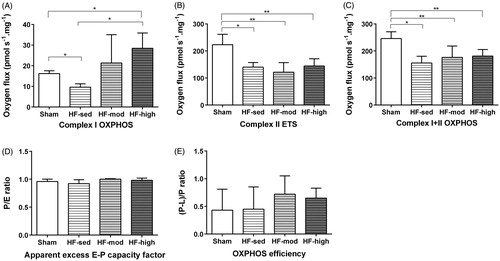
We measured both coupled oxidative phosphorylation (OXPHOS) and uncoupled OXPHOS (or electron transfer capacity; ETS). The ratio of ETS to OXPHOS (E/P ratio) for the combined CI + CII substrate state was calculated with no difference between groups, indicating no excess capacity of the ETS (). To describe the coupling efficiency with the substrates malate and glutamate, we corrected OXPHOS capacity for LEAK respiration ((P–L)/P ratio), which showed no significant difference among the subgroups ().
Discussion
This study investigated the effect of moderate and high-intensity exercise training on myocardial energy metabolism in HF. Compared to sham controls, failing hearts had a significantly lower PCr/ATP ratio, which has been used as a biomarker of myocardial function [Citation14,Citation27] (). The lower PCr/ATP level resulted from a lower level of PCr () in the face of unchanged ATP levels (). Exercise training induced a relative increase in the level of ATP in HF rats () with no change in PCr/ATP ratio (). Furthermore, the reduced mitochondrial respiratory capacity in HF rats compared to Sham partially recovered by high-intensity exercise training, through increased complex I respiration. These adaptations were associated with improved VO2max and running capacity.
High-intensity exercise training may provide an important improvement in cardiac performance [Citation28–31]. We have previously reported a significant decrease in cardiomyocyte hypertrophy and an increase in contractility after high-intensity interval training [Citation32]. We also found enhanced cardiac performance in rats with HF after high intensity exercise training, as indicated by the increased VO2max and improved myocardial contractile function (Supplementary Figure S1).
We hypothesized that high-intensity exercise would increase PCr/ATP ratio, as Perseghin and colleagues [Citation33] observed an increased PCr/ATP ratio associated with an increased diastolic function in middle-aged athletes compared to sedentary counterparts. Contrary to this, we observed no PCr/ATP recovery () in HF rats after high-intensity exercise. However, other randomized clinical trials are in accordance with our results, where improved cardiac performance in patients with diverse conditions of heart disease after low- to moderate intensity training was achieved without changes in PCr/ATP ratio [Citation34,Citation35]. In these studies, the authors suggested that the improved cardiac function was not caused by improved cardiac energetics, and that PCr/ATP ratio is not a suitable biomarker to evaluate the recovery of cardiac performance in HF patients after an exercise training program.
Mitochondrial complex I activity is responsible for the transfer of electrons from NADH to co-enzyme Q (), and plays an important role in oxidative phosphorylation and ATP synthesis. In line with a previous study [Citation36], we found a significant improvement in complex I activity after high-intensity exercise training in HF rats (). Their increased ATP level () was probably due to enhanced oxidative phosphorylation associated with improved complex I activity.
Figure 4. Effect of heart failure (HF, red arrow) and exercise training (green arrow) on VO2max, mitochondrial complexes function, ATP, PCr, and PCr/ATP ratio. Upwards and downwards arrows represent relative increase or decrease from baseline, respectively. Photo at the upper left corner depicts running rat and apparatus for VO2max measurement on treadmill. Oxidative phosphorylation is illustrated in cardiac mitochondria. Electrons transfer from NADH to oxygen (light green pathway) through respiratory chain (from mitochondrial complexes I–IV). The transfer generates an electrochemical gradient of a proton (H+) across the inner mitochondrial membrane. This gradient drives ATP synthesis by complex V (ATP synthase). ATP synthesized by oxidative phosphorylation is transported by ANT. High energy phosphor bond is transferred within ATP and PCr by creatine kinase energy shuttle (mtCK and mmCK). ATP synthesized at mmCK in the cytosol is consumed by ATPase and releases energy for cardiac muscle contractile work. ADP: adenosine diphosphate; ATP: adenosine triphosphate; ANT: adenine nucleotide translocator; ATPase: adenosine triphosphatase; CoQ: co-enzyme Q; CytC: cytochrome C; Cr: creatine; H+: proton; e−: electron; FAD/FADH2: flavin adenine dinucleotide; mmCK: myofibrillar creatine kinase; mtCK: mitochondrial creatine kinase; NAD/NADH: nicotinamide adenine dinucleotide; Pi: inorganic phosphate; PCr: phosphocreatine.
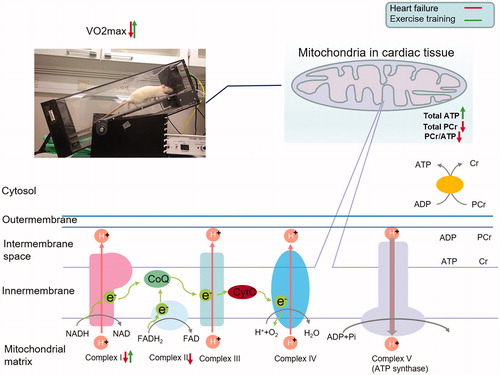
An alternative ATP synthesis pathway in cardiac tissues is the conversion of PCr to ATP. This conversion requires functional proteins, among which creatine kinase (CK) serves as the most pivotal isoenzyme that mediates the transfer of high energy phosphate bonds within the two metabolites (). Both mitochondrial CK (mtCK) and myofibrillar CK (mmCK) act as a buffering system, promoting the conversion of PCr to ATP in the cytosol, to sustain a stable ATP concentration. The imbalance between ATP synthesis and the activities of mtCK and mmCK may explain the depletion of PCr in the HF rats. Exercise training did not recover the non-equilibrium level of ATP and PCr in HF rats, but instead caused increased ATP levels, probably through increased complex I activity. Abnormal activity of CK enzymes has been reported in HF [Citation7,Citation27,Citation37]. Kemi and colleagues found that the mmCK activity in HF was significantly increased (recovered to the same level of with sham group) after exercise training, whereas mtCK activity remained unchanged [Citation7]. Their findings suggested that dysfunctional mtCK in the HF rats could be a possible explanation for the failed PCr recovery, even in the presence of increased ATP accumulation in HF-sed after the exercise training intervention.
Limitations
In this study, we were only able to use whole body oxygen consumption as a measure of in vivo metabolism, which is not entirely dependent on cardiac function but also the ability of the blood to carry oxygen and the skeletal muscles ability to utilize oxygen. Furthermore, permeabilized fibers from cryopreserved muscle were used to measure the mitochondrial respiratory. Even though samples from different groups were handled identically and an interaction effect between treatment and cryopreservation seems unlikely, comparison of mitochondrial respiration values with other studies that did not perform cryopreservation is limited. In particular, our respiration values appear reduced compared to studies in which no cryopreservation was used [Citation38–42].
Conclusion
In the experimental model of chronic HF in rats, regular exercise training improves cardiac function and increases VO2max and exercise capacity. This study demonstrated that these changes are associated with higher ATP levels and increased complex I activity in the myocardium, but no changes in the PCr/ATP ratio. These observations indicate that the PCr/ATP ratio is not suited as a biomarker to evaluate the beneficial effects of exercise training in the heart. Although previous studies suggest that failure to enhance energy tranfer by the mitochondrial creatine kinase might be a limiting factor for PCr synthesis, the mechanism remained unclear. The exact mechanisms of mitochondrial biology in HF should be further investigated to explore the impact of exercise training on ATP levels and CI respiration.
Supplemental Material
Download Zip (542.8 KB)Acknowledgments
We thank Karin Garten and Ragnhild Elisabeth Nyhus Røsbjørgen for their laboratory assistance. MR spectroscopy was performed at the MR Core Facility, NTNU, Norwegian University of Science and Technology. MR core facility is funded by the Faculty of Medicine at NTNU and Central Norway Regional Health Authority.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584.
- McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–1889.
- James S, Barton D, O'Connell E, et al. Life expectancy for community-based patients with heart failure from time of diagnosis. Int J Cardiol. 2015;178:268–274.
- Savage PA, Shaw AO, Miller MS, et al. Effect of resistance training on physical disability in chronic heart failure. Med Sci Sports Exerc. 2011;43:1379–1386.
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221–1238.
- Negrao CE, Middlekauff HR, Gomes-Santos IL, et al. Effects of exercise training on neurovascular control and skeletal myopathy in systolic heart failure. Am J Physiol Heart Circ Physiol. 2015;308:H792–802.
- Kemi OJ, Hoydal MA, Haram PM, et al. Exercise training restores aerobic capacity and energy transfer systems in heart failure treated with losartan. Cardiovasc Res. 2007;76:91–99.
- Bruning RS, Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Prog Cardiovasc Dis. 2015;57:443–453.
- Hollriegel R, Winzer EB, Linke A, et al. Long-term exercise training in patients with advanced chronic heart failure: sustained benefits on left ventricular performance and exercise capacity. J Cardiopulm Rehabil Prev. 2016;36:117–124.
- Achterberg PW, Weiss RG. Regional myocardial metabolism of high-energy phosphates in patients with coronary artery disease. N Engl J Med. 1991;324:1218–1219.
- Liu JB, Wang CS, Murakami Y, et al. Mitochondrial ATPase and high-energy phosphates in failing hearts. Am J Physiol-Heart Circul Physiol. 2001;281:H1319–H1326.
- Casademont J, Miro O. Electron transport chain defects in heart failure. Heart Failure Rev. 2002;7:131–139.
- Li P, Wang B, Sun F, et al. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci Rep. 2015;5:11.
- Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818.
- Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196.
- Schocke MF, Esterhammer R, Arnold W, et al. High-energy phosphate metabolism during two bouts of progressive calf exercise in humans measured by phosphorus-31 magnetic resonance spectroscopy. Eur J Appl Physiol. 2005;93:469–479.
- Greiner A, Esterhammer R, Messner H, et al. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg. 2006;43:978–986.
- Stølen T, Høydal MA, Ahmed MS, et al. Exercise-induced changes in micro-RNA profile is associated with improved cardiac function and electrophysiology in rats with heart failure after myocardial infarction. Cardiovasc Res. 2019.
- Loennechen JP, Stoylen A, Beisvag V, et al. Regional expression of endothelin-1, ANP, IGF-1, and LV wall stress in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2001;280:H2902–10.
- Johnsen AB, Hoydal M, Rosbjorgen R, et al. Aerobic interval training partly reverse contractile dysfunction and impaired Ca2+ handling in atrial myocytes from rats with post infarction heart failure. PloS One. 2013;8:e66288.
- Wisloff U, Helgerud J, Kemi OJ, et al. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–10.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Esmaeili M, Moestue SA, Hamans BC, et al. In vivo ³¹P magnetic resonance spectroscopic imaging (MRSI) for metabolic profiling of human breast cancer xenografts. J Magn Reson Imag. 2015;41:601–609.
- Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152.
- Kuznetsov AV, Veksler V, Gellerich FN, et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976.
- Kuznetsov AV, Kunz WS, Saks V, et al. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem. 2003;319:296–303.
- Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA. 2005;102:808–813.
- Kemi OJ, Haram PM, Loennechen JP, et al. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–172.
- Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. A randomized study. Circulation. 2007;115:3086–3094.
- Haram PM, Kemi OJ, Lee SJ, et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81:723–732.
- Ellingsen O, Halle M, Conraads V, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135:839–849.
- Wisloff U, Loennechen JP, Currie S, et al. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162–174.
- Perseghin G, De Cobelli F, Esposito A, et al. Left ventricular function and energy metabolism in middle-aged men undergoing long-lasting sustained aerobic oxidative training. Heart. 2008;95:630–635.
- Beer M, Wagner D, Myers J, et al. Effects of exercise training on myocardial energy metabolism and ventricular function assessed by quantitative phosphorus-31 magnetic resonance spectroscopy and magnetic resonance imaging in dilated cardiomyopathy. J Am Coll Cardiol. 2008;51:1883–1891.
- Holloway CJ, Dass S, Suttie JJ, et al. Exercise training in dilated cardiomyopathy improves rest and stress cardiac function without changes in cardiac high energy phosphate metabolism. Heart. 2012;98:1083–1090.
- Kraljevic J, Marinovic J, Pravdic D, et al. Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart. Cardiovasc Res. 2013;99:55–64.
- Nascimben L, Friedrich J, Liao R, et al. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation. 1995;91:1824–1833.
- Power AS, Norman R, Jones TLM, et al. Mitochondrial function remains impaired in the hypertrophied right ventricle of pulmonary hypertensive rats following short duration metoprolol treatment. PloS One. 2019;14:e0214740.
- Beaudoin MS, Perry CG, Arkell AM, et al. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. J Physiol. 2014 ;592:2519–2533.
- MacDonald JR, Oellermann M, Rynbeck S, et al. Transmural differences in respiratory capacity across the rat left ventricle in health, aging, and streptozotocin-induced diabetes mellitus: evidence that mitochondrial dysfunction begins in the subepicardium. Am J Physiol, Cell Physiol. 2011;300:C246–55.
- Lin A, Krockmalnic G, Penman S. Imaging cytoskeleton-mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci USA. 1990;87:8565–8569.
- Veksler VI, Kuznetsov AV, Sharov VG, et al. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196.
