Abstract
Objective. Patients with diabetes have higher mortality rate than patients without diabetes after ST-segment elevated myocardial infarction (STEMI). Prognosis of patients with new onset diabetes (NOD) after STEMI remains unclear. The aim of this study was to evaluate the prognosis of patients with NOD compared to that of patients without NOD after STEMI. Design. This study was a retrospective observational study. We enrolled 901 STEMI patients. Patients were divided into diabetic and non-diabetic groups at index admission. Non-diabetic group was divided into NOD and non-NOD groups. Kaplan-Meier analysis and Cox's proportional hazard regression models were used to compare major adverse cardiac events (MACE) free survival rate and hazard ratio for MACE between NOD and non-NOD groups. Results. Mean follow-up period was 59 ± 28 months. Diabetes group had higher MACE than non-diabetes group (p = .038). However, MACE was not different between NOD and non-NOD groups (p = 1.000). After 1:2 propensity score matching, incidence of MACE was not different between the two groups. In Kaplan-Meier survival curves, MACE-free survival rates were not statistically different between NOD and non-NOD groups either (p = .244). Adjusted hazard ratios of NOD for MACE, all-cause of death, recurrent myocardial infarction, and target vessel revascularization were 0.697 (95% confidence interval [CI]: 0.362–1.345, p = .282), 0.625 (95% CI: 0.179–2.183, p = .461), 0.794 (95% CI: 0.223–2.835, p = .723), and 0.506 (95% CI: 0.196–1.303, p = .158), respectively. Conclusion. This retrospective observational study with a limited statistical power did not show a different prognosis in patients with and without NOD.
Objective
Diabetes is one major risk factor for development of cardiovascular disease. Risk of cardiovascular disease in patients with diabetes is two to three times higher than that in patients without diabetes [Citation1]. Diabetes is also a strong risk factor for cardiovascular events after acute myocardial infarction (MI) [Citation2]. Prognosis of patients with new onset diabetes (NOD) after ST-segment elevated myocardial infarction (STEMI) is currently unclear. We hypothesized that NOD could increase the incidence of cardiovascular events than non-NOD, because DM contributes to cardiovascular events compared to non-DM. Previous studies have shown that β-blockers and statins are associated with the development of diabetes [Citation3,Citation4]. The guidelines for management of STEMI recommend the use of β-blockers and statins for secondary prevention [Citation5]. The aim of this study was to evaluate the prognosis of patients with NOD, as compared to that of patients without NOD in a population without diabetes at the time of the index SETMI who were treated with standard drug therapy after successful primary percutaneous coronary intervention (PCI).
Design
Study design
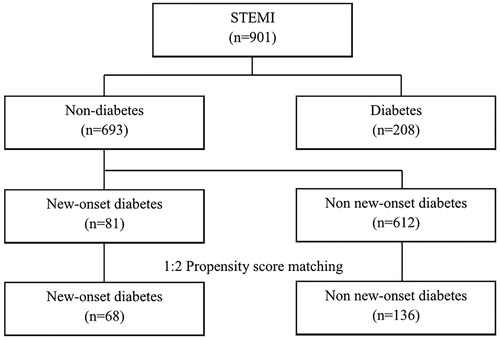
This study was a retrospective observational cohort study. We consecutively enrolled 30-day survivors after STEMI who underwent primary PCI successfully from 2003 to 2009. Nine hundred and one patients (716 males, 58 ± 13 years old) were enrolled. All clinical data were obtained from electronic medical records (EMR) according to the principles of the Declaration of Helsinki [Citation6]. Individual patient’s data were linked via personal identification number. We protected the privacy of patients and the confidentiality of their personal information according to the Guideline of medical ethics in Korea [Citation7] and Bioethics and Safety Act in Korea [Citation8]. Information of events including follow-up loss and death of patients was collected from the National Health Insurance Service in Korea or by direct contacting the patient through phone. All patients were divided into two groups: those who had diabetes (n = 208, 154 males; 59 ± 11 years old) and those who did not have diabetes (n = 693, 562 males; 57 ± 12 years old) at index STEMI. Diabetes status of patients was judged by their fasting blood glucose levels or medical history. Patients without diabetes at index admission were classified into NOD group (n = 81, 67 males; 57 ± 12 years old) and non-NOD group (n = 612, 495 males; 57 ± 12 years old) depending on the development of NOD (). NOD was defined as diabetes newly diagnosed at least one year after STEMI in a population without diabetes at the time of the index admission. We diagnosed NOD when fasting plasma glucose level ≥7.0 mmoles/l or random plasma glucose level ≥11.1 mmoles/l and/or hemoglobin A1C ≥6.5% according to the American Diabetes Association Guidelines [Citation9].
Study end-points
Primary end-point was major adverse cardiac events (MACEs), including all-cause of death, recurrent MI, and target vessel revascularization (TVR). Secondary end-point was each component of MACEs. Primary and secondary end-points were compared between the NOD group and the non-NOD group.
Statistical analysis
IBM SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Continuous variables were expressed as means ± standard deviation. They were compared by independent t-test. Categorical variables were expressed as numbers and percentage. They were compared with Chi-square test. To adjust differences in baseline characteristics between the NOD group and the non-NOD group, propensity score matching (1:2) was performed (). Age, gender, hypertension, smoking, dyslipidemia, family history of cardiovascular disease, and left ventricular ejection fraction (LVEF) were used as covariates for matching. There are other prognostic risk factors for prognosis of post myocardial infarction [Citation10,Citation11]. However, since we reviewed retrospectively patient’s EMR, we selected covariates recorded for all patients on EMR. After propensity score matching, cumulative incidences of primary and secondary end points were estimated by Kaplan–Meier curve. Separation of curves was tested with log-rank test. Cox’s proportional hazard model was used to assess adjusted relative hazard ratio of NOD to the study end point. Age, hypertension, smoking, dyslipidemia, family history of cardiovascular disease, history of cerebrovascular disease, use of β-blocker, statin, angiotensin blockade agents, calcium channel blocker, diseased vessels, Killip class, and LVEF were used as adjusted covariates for the Cox’s proportional hazard model. Results of multivariate analysis are expressed as adjusted hazard ratio with 95% confidence intervals for clinical outcomes. A two-sided p value of less than .05 was regarded as statistically significant. PASS 14 (NCSS, LLC. Utah, USA) was used for sample size. The sample size was calculated to achieve 80% power at a .05 significance level when the hazard ratio is actually .800. The number of events required to achieve this power is 709.6. It is anticipated that proportions of subjects having the events during the study is .200 for the control group and .200 for the treatment group. These results assume that the hazard ratio is constant throughout the study and that Cox proportional hazard regression is used to analyze the data.
Results
Of 901 patients, 208 (23%) had diabetes at admission. Patients with diabetes were older than patients without diabetes. Patients with diabetes had higher rates of hypertension, end-stage renal disease, and multi-vessel disease but lower rates of smoking than patients without diabetes. Other baseline characteristics were not different between the diabetes group and the non-diabetes group (Supplementary Table 1). During mean follow-up period of 59 ± 28 months (range, 6 to 156 months), 81 (12%) patients of the non-diabetes group (n = 693) were diagnosed as NOD. The NOD group (n = 81, 54 ± 7 years old) was older than the non-NOD group (n = 612, 52 ± 12 years old). Other baseline characteristics were not different between the two groups (Supplementary Table 2). There are only about 66% of STEMI patients were prescribed statins at hospital discharge and the proportion of NOD patients was even less (59% in NOD, 67% in non-NOD). However, this was not meaningful statistically (p-value=.167). After 1:2 propensity score matching, the NOD group (n = 68, 54 ± 11 years old) and the non-NOD group (n = 136, 53 ± 12 years old) had no difference in baseline characteristics (). After diagnosis of NOD, oral antidiabetic drugs were prescribed for 61 patients (90%) of the 68 NOD patients, no antidiabetic medication were prescribed for 6 patients (9%) and just life style modifications were recommended. For only 1 patient (1%), insulin was prescribed. Incidences of MACE and all-cause of death in patients with diabetes were higher than those in patients without diabetes (MACE: 31.7% vs. 24.5%, p = .038; all-cause of death: 20.2% vs. 13.7%, p = .022, Supplementary Table 3). The incidence of all-cause of death in the non-NOD group was higher than that in the NOD group (15% vs. 5%, p = .015, Supplementary Table 4). However, MACEs or other secondary end-points did not show statistical differences between NOD and non-NOD groups. After 1:2 propensity score matching, primary or secondary end-points were not different between NOD and non-NOD groups (). Although there were no definite criteria of time to define ‘early NOD’ or ‘late NOD’, we defined the ‘early NOD’ (n = 32) as NOD was diagnosed within 1 year after index STEMI and the ‘late NOD’ (n = 36) was diagnosed 1 year after index STEMI. The primary and secondary endpoints were not different between early NOD and late NOD (). Among non-diabetes group (n = 204), number of patients with killip class 4 were 6. Three out of six patients were diagnosed with NOD. All 3 NOD patients did not have MACE. In Kaplan–Meier survival curve and Cox’s proportional hazard model, there were no statistical differences in event-free survival rates or hazard ratio between the two groups (, ).
Figure 2. Kaplan-Meier survival curves for free of adverse outcomes in NOD group and non-NOD groups. MACEs: Major adverse cardiac events; MI: Myocardial infarction; NODM: New-onset diabetes.
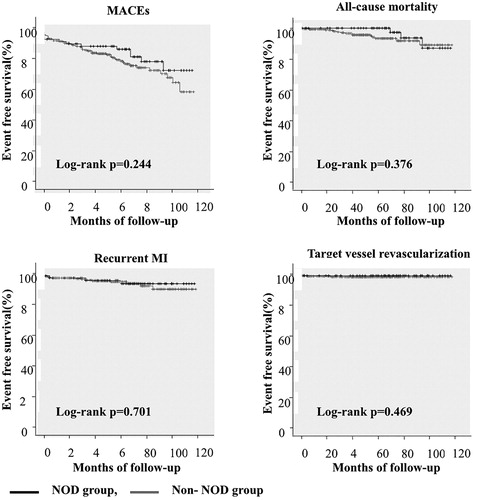
Table 1. Baseline clinical characteristics after 1:2 propensity score matching of non-diabetes patients at index admission.
Table 2. Clinical outcomes of NOD group and non-NOD group after 1:2 propensity score matching.
Table 3. Clinical outcomes of early NOD group and late NOD group.
Table 4. Cox’s proportional hazard model (95% CI).
Discussion
The prognosis of diabetic patients was worse than that of non-diabetic patients in our study, similar to previous studies [Citation12,Citation13]. It is known that hyperglycemia inhibits the production of nitric oxide in endothelial and vascular smooth muscle cells. It can induce vasoconstriction of vessel and thrombotic condition by reducing antiplatelet prostanoid prostacyclin. With insulin resistance, ischemic myocardium is forced to use free fatty acids as energy source. This has a negative effect on myocardial cellular function by lowering LVEF [Citation14,Citation15]. In an observational study of 7820 hyperglycemic patients with acute myocardial infarction, lower glucose level has been found to be associated with better prognosis [Citation16]. DIGAMI [Citation17] and HI-5 trial [Citation18] have demonstrated that intensive blood glucose control can reduce mortality. Based on these previous studies, we hypothesized that patient without NOD after STEMI might have better prognosis compared to patients with NOD. Contrary to our hypothesis, the prognosis of patients with NOD was not poorer than that of patients without NOD in the present study. This result may suggest that NOD did not directly increase mortality rate. However, the statistical power to detect a difference in primary outcome of this study was only 18.0%. Our study had a mean follow-up period of around 5 years which was relatively short to confirm macrovascular complications of diabetes. However, macrovascular complications such as cardiovascular disease of patients with diabetes are not closely related to the duration of diabetes [Citation19]. In this study, 77% of patients diagnosed with NOD were taking β-blockers and 59% of them were taking statins. β-blockers can reduce the risk of death by reducing myocardial oxygen demand, remodeling of left ventricle, and increasing coronary blood flow [Citation20]. Statins can improve endothelial function and coronary flow [Citation21]. These beneficial effects of β-blockers and statins might be emphasized besides their association with diabetes. According to the result of this study, we tried to emphasize the importance of standard therapy for secondary prevention without hesitation of using β-blockers or statins, which are known to be associated with NOD. We could not confirm this importance because the statistical power is too low. However, we accepted the tendency that the prognosis of NOD was not poor considering that the sample size was too small in this retrospective study.
Limitations
Our study had some limitations. First, since this was a retrospective study, we could only confirm the blood glucose status of patients after STEMI as routine laboratory test results performed at outpatient clinic. As some patients were diagnosed as NOD at other hospital, it was not possible to evaluate the exact duration of glucose abnormality and the extent of glucose derangement. As the non-diabetes patients did not undergo the oral glucose tolerance test in the present study, we could not check the impaired glucose tolerance status. Although patients with impaired glucose tolerance but normal fasting glucose level might have worse prognosis than patients without impaired glucose tolerance [Citation22,Citation23], we could not evaluate the prognosis of these patients. Second, this was a single center observational study. Thus, there might have been selection bias. Third, this study had low statistical power because the sample size was too small. To have meaningful statistical power, this study should have a sample size at least 3548. Because this study was a retrospective study, it was not possible to enroll more patients. We thought that these might be effect to clinical outcome. Fourth, our study had a relatively short follow-up period to see diabetic complications. However, macrovascular complications are less likely to be associated with the duration of diabetes compared to microvascular complications. Fifth, we did not evaluate whether β-blockers and statins might contribute to the development of diabetes directly. Sixth, we did not evaluate heart failure as a clinical outcome. It is known that heart failure is a major source of mortality after myocardial infarction [Citation24]. Therefore, future studies are needed to evaluate the occurrence of heart failure as a clinical outcome in NOD patients.
Conclusion
Diabetes increases cardiovascular mortality. However, this study showed that NOD does not directly increase MACEs after STEMI. Although β-blockers and statins are associated with diabetes, effective standard care and strict follow-up monitoring must be done to reduce mortality rate after STEMI. We tried to emphasize again the importance of secondary prevention therapy and confirm the importance of proper monitoring. However, this study has too small sample size and short-term periods. We consider that a prospective study with larger number of patients and longer follow-up periods is needed to confirm this study.
Supplemental Material
Download Zip (50.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bartnik M, Malmberg K, Norhammar A, et al. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004;25(22):1990–1997.
- Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–942.
- Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742.
- Fonseca V, Sharma PP, Shah M, et al. Risk of new-onset diabetes mellitus associated with beta-blocker treatment for hypertension. Curr Med Res Opin. 2011;27(4):799–807.
- Ibanez B, James S, Antunes MJ, ESC Scientific Document Group, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Korean Med Assoc. 2014;57(11):899–902.
- Kim O-J, Park YH, Hyun BG. Development of the codes and guidelines of medical ethics in Korea. J Korean Med Assoc. 2017;60(1):8–17.
- Ministry of Government Legislation [Internet]. South Korea’s Bioethics and Safety Act; 2008. June. Available from: http://www.moleg.go.kr/english/korLawEng?pstSeq=47518.
- Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diab Care. 2015;38(9):1777–1803.
- Jemberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36(19):1163–1170.
- Wang Y, Li J, Zheng X, et al. Risk Factors associated with major cardiovascular events 1 year after acute myocardial infarction. JAMA Netw Open. 2018;1(4):e181079.
- Timmer JR, van der Horst IC, Henriques JP, et al. Long-term clinical outcome of ST-segment elevation myocardial infarction patients with and without diabetes mellitus in the Zwolle trial. Neth Heart J. 2003;11(10):387–393.
- Nesto RW, Zarich S. Acute myocardial infarction in diabetes mellitus. Circulation. 1998;97(1):12–15.
- Gibson CM, Ryan KA, Murphy SA, et al. Impaired coronary blood flow in nonculprit arteries in the setting of acute myocardial infarction. The TIMI Study Group. Thrombolysis in myocardial infarction. J Am Coll Cardiol. 1999;34(4):974–982.
- Gardner GS, Frisch DR, Murphy SA, et al. Effect of rescue or adjunctive percutaneous coronary intervention of the culprit artery after fibrinolytic administration on epicardial flow in nonculprit arteries. Am J Cardiol. 2004;94(2):178–181.
- Kosiborod M, Inzucchi SE, Krumholz HM, et al. Glucose normalization and outcome in patients with acute myocardial infarction. Arch Intern Med. 2009;169(5):438–446.
- Malmberg K, Rydén L, Wedel H, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26((7):650–651.
- Cheung NW, Wong VW, McLean M. Hyperglycemia: intensive insulin infusion in infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care. 2006;29(4):765–770.
- Lee HR, Yu JM, Choi MG, et al. Risk factors for early development of macrovascular complications in Korea Type 2 diabetes. Korean Diabetes J. 2009;33(2):134–142.
- López-Sendón J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25(15):1341–1362.
- Patti G, Cannon CP, Murphy SA, et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation. 2011;123(15):1622–1632.
- Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359(9324):2140–2144.
- Bronisz A, Kozinski M, Magielski P, et al. Value of oral glucose tolerance test in the acute phase of myocardial infarction. Cardiovasc Diabetol. 2011;10(1):21.
- Torabi A, Cleland JG, Rigby AS, et al. Development and course of heart failure after a myocardial infarction in younger and older people. J Geriatr Cardiol. 2014;11(1):1–12.