Abstract
Objectives. To investigate nationwide changes in procedure rates, patient selection, and prognosis after all surgical aortic valve replacements. Design. Patients undergoing primary surgical aortic valve replacement between 2001 and 2016 were identified from three nationwide registers with compulsory reporting to examine trends in aortic valve surgery over four four-year time periods. Results. A total of 12,139 surgical aortic valve replacement procedures (mean age 61.9 ± 11.8 years, 39.1% women) were performed. The total number of biological valves increased from 1001 (42.9%) to 2526 (75.5%) from 2001–2004 to 2013–2016 (p < .001). During the first and last time periods the comorbidity burden increased; share of patients with hypertension increased from 37.5% to 46.9% (p < .001), diabetes from 14% to 16.5% (p = .01) and previous stroke from 5.2% to 7.2% (p = .01). The proportion of women undergoing surgery decreased from 40% to 36.1% from 2001–2004 to 2013–2016, respectively (p = .01). Overall 28-day mortality was 3.5%. In patients with biologic valve the multivariable-adjusted risk of short-term mortality decreased steadily in every four-year period from 2001–2004 to 2005–2008 (HR, 0.66; 95% CI 0.47–9.92), 2009–2012 (HR, 0.54; 95% CI, 0.39–0.75) and 2013–2016 (HR, 0.41; 95% CI, 0.29–0.58), whereas short-term mortality remained similar in patients with mechanical valve. The risk of four-year postoperative mortality after all surgical aortic valve replacements stayed constant. Conclusions. The use of biologic aortic valve prosthesis has increased from 2001 to 2016. The proportion of women has declined markedly. The short-term mortality has decreased and the long-term mortality has stayed constant despite increasing comorbidity burden.
Introduction
Surgical aortic valve replacement (SAVR) with either mechanical or biologic valve is the most common type of valvular surgery in western countries [Citation1]. The main indications for surgery are aortic valve stenosis and aortic valve insufficiency, with the former being more common [Citation1]. The prevalence of aortic valve stenosis is estimated to be 0.3–0.7% and insufficiency 0.3–0.5% [Citation2,Citation3]. Moreover, their prevalence is markedly higher in population >75 years; 2.8% and 2.0%, respectively [Citation3]. The prevalence of aortic stenosis is continually increasing, partly due to an increasingly aging population [Citation4]. However, despite patients being older and presenting with increased comorbidities, the overall mortality rates following cardiac surgery have steadily declined [Citation4–6].
The selection of prosthesis type for valve replacement is based on balancing the risks of life-long anticoagulation with mechanical and reoperation with bioprosthetic valves. Due to their limited durability, bioprosthesis are recommended for the elderly [Citation1,Citation7,Citation8]. The data on survival after SAVR is still controversial. Recently Goldstone et al. [Citation9] showed that SAVR with mechanical prosthesis was associated with lower mortality among patients aged up to 55 years, whereas in a study by Glaser et al. [Citation7], patients aged 50–69 years survived better with mechanical valves compared to biological. In contrast, Chiang et al. found no difference in mortality [Citation10]. Hence, it has been suggested that a biologic prosthesis could be suitable also for younger patients.
The aim of the present study was to examine nationwide trends in SAVR procedures between 2001 and 2016 using Finnish nationwide register data. We assessed longitudinal trends in valve type and patient selection, the short-term 28-day prognosis and long-term 4-year prognosis during the study period.
Methods
Data sources and study population
The data for Finnish Cardiovascular Diseases Register was formed by combining individual patient data from three nationwide electronic health care registers: The National Hospital Discharge Register, the National Drug Reimbursement Register, and the Causes of Death Register. The information on diagnoses and procedures was collected from the National Hospital Discharge Register which also includes a separate detailed section for invasively treated cardiac patients. The data on drug purchases for reimbursed medications was gathered from the Drug Reimbursement Register. In addition, the information on the causes of death was added from the National Causes of Death Register.
The Hospital Discharge register contains diagnoses for each secondary and tertiary care patient visit and Causes of Death Registers contain diagnoses of underlying, contributing, or immediate causes of death. The recording of the diagnoses to the registers is obligatory and done by the treating physicians using the codes from the International Classification of Diseases 10th revision (ICD-10). For the present study, we used data from the years 2001 through 2016. The coverage of the Finnish Cardiovascular Diseases Register data is shown to be good, for revascularization procedures it was previously shown to be over 90% [Citation11].
Between 2001 and 2016, 12,146 patients had undergone first SAVR with or without concomitant coronary artery bypass grafting (CABG) surgery in Finland. We divided the patients into three groups by procedure type: 1) all SAVR procedures; 2) mechanical prosthesis; and 3) biologic prosthesis.
A patient was considered to have diabetes, hypertension, or chronic lung disease (i.e., asthma or chronic obstructive pulmonary disease) if a specific medication was found in the Drug Reimbursement Register or the ICD codes matched with these diseases prior the operation [Citation6]. The operation was defined as urgent if it was necessary to perform within one week of arrival to the hospital. Before 2003 the information about urgency was not available in the registers, whereas all procedures in which the patient had arrived in the hospital through the emergency room were defined as urgent. Endocarditis was not an exclusion criteria, so all the endocarditis patients are included to the population.
Follow-up and outcomes
The follow-up ended on December 31, 2016. The outcome events were detected through National Hospital Discharge and Causes of Death Registers. These registers are nationwide and the coverage of follow-up is practically 100%. Only persons who have permanently moved abroad are lost to follow-up. On average, 0.1–0.2% of the Finnish population move abroad each year but this proportion is likely to be even lower among elderly cardiac patients.
Four endpoints were used: 28-day all-cause postoperative mortality, 4-year all-cause postoperative mortality, 4-year incidence of cardiovascular events and 4-year risk of intracranial bleeding. Cardiovascular mortality was defined as mortality related to disease of the circulatory system (ICD-10 codes I20-25, I46, R96, R98, I61, I63, and I64) as the underlying, contributing, or immediate cause of death. The intracranial bleeding was defined as cerebral bleeding or nontraumatic intracranial bleeding (I61 and I62), subarachnoidal hemorrhage was not included. Myocardial infarction was defined with ICD-10 codes I21 and I22 as a hospital discharge diagnosis or as the underlying, contributing or immediate cause of death. Stroke, excluding subarachnoid hemorrhage, was defined with ICD-10 codes I61 and I63 (not I63.6) as a hospital discharge diagnosis or as the underlying, contributing or immediate cause of death. The validity of coronary, stroke, and heart failure diagnoses in the Finnish registers has been previously fully described [Citation11–13].
Statistical methods
To assess the longitudinal changes in procedure types, patient characteristics, and post–procedural outcomes, we divided the study period into four four-year categories by the year of the SAVR operation: 2001–2004, 2005–2008, 2009–2012, and 2013–2016. Trends in the patient characteristics across the time strata were compared using the Cochran-Armitage trend test for categorical variables and regression analysis for continuous variables. We used Cox proportional hazards regression models with follow-up truncated at four years to estimate the hazard ratios for post-procedural mortality and cardiovascular events in different time periods. In addition, Cox proportional hazards regression was used to assess the 28-day post-procedural hazard of all-cause mortality in the different time periods. Proportional hazard assumptions were evaluated graphically through plotting the Schoenfeld residuals and no strong evidence against proportionality were obtained. The period 2001–2004 was used as the reference category in all models. The models were adjusted for sex, age, urgency of the surgery, diabetes (yes/no), hypertension (yes/no), chronic lung disease (yes/no), previous myocardial infarction (yes/no), and previous stroke (yes/no).
Results
Trends in SAVR rates and types
In total, 12,139 SAVRs were performed in Finland years 2001–2016 (). The incidence of SAVRs in Finland during the whole study period of 16 years was 14 per 100,000 person-days. Over the study period, there was an increasing trend to using biologic valves (p < .001) whereas the use of mechanical valves markedly decreased (p < .001) (). During the first time period 2001–2004, the proportion of biologic valves was 42.9% and their proportion increased steadily to 75.5% in 2013–2016. Altogether 16.5% of all SAVR procedures were classified as urgent. Urgent procedures increased significantly over the study period (p < .001) (). However, the amount of urgent procedures stayed rather constant after the first two 4-year periods. When biologic and mechanical valve groups were analyzed separately the increase in urgent procedures was only seen in the mechanical valve group (p < .001). The proportion of concomitant CABG procedures has increased from 22% to 9.5% (p < .001) from 2001–2004 to 2013–2016 (). The same change is also seen with mechanical and biological valves; from 16% to 4.4% (p < .001) and 30% to 11.1% (p < .001), respectively ().
Table 1. Trends in patient selection for surgical aortic valve replacements in Finland in 2001–2016.
Trends in patient selection
Trends in patient selection for SAVR are presented in . The mean age of all SAVR patients was 69.1 years, staying constant over the 16-year period. The mean age of patients undergoing mechanical and biological SAVR remained unchanged; 59.5 (11.8) years and 74.7 (7.3) years, respectively. During the whole study period, the average proportion of women receiving an aortic valve prosthesis was 39.1%. The proportion of women decreased from 2001 to 2016 (p = .01) and this trend was apparent in both mechanical and biologic SAVR groups (p < .001 for both) (, ). The decrease in the proportion of women increased during the last four-year time period (). Biologic prosthesis was the more common choice for women; in the mechanical valve group, the proportion of women was 27.7% and in the biologic group 45.7%, respectively. On average, 72.8% of the patients had aortic valve stenosis and the proportion stayed constant during the study period ().
Figure 1. Decreasing proportion of women in the surgical aortic valve operations with mechanical and biologic prosthesis.
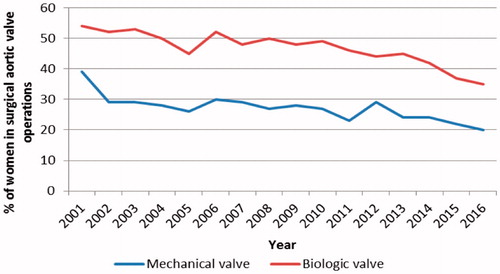
The comorbidity burden of the SAVR patients increased during the 16 years. Overall, the share of patients with hypertension (p < .001), diabetes (p = .01), and previous stroke (p = .01) increased (). However, the prevalence of previous myocardial infarction declined slightly (p = .05), particularly in the mechanical valve group (p < .001). In separate analyses, none of the comorbidities showed an increase in the mechanical valve group, while in the biologic valve group we observed an increased trend of patients with hypertension (p = .01) ().
Trends in 28-day mortality after SAVR
The hazard ratios (HR) for all-cause mortality within 28 days after SAVR are presented in . During the whole study period the short-term mortality for all SAVR procedures was 3.5%. The short-term mortality decreased from 2001–2004 to 2009–2012; 153/100,000 to 127/100,000 respectively (HR, 0.61; 95% CI, 0.46–0.80) and from 2001–2004 to 2013–2016; 153/100,000 to 89/100,000, respectively (HR, 0.47; 95% CI, 0.35–0.63). In patients who underwent a bioprosthesis operation, the short-term mortality decreased steadily in every four-year period from 2001–2004 to 2005–2008 (HR, 0.66; 95% CI 0.47–9.92), 2009-2012 (HR, 0.54; 95% CI, 0.39–0.75) and 2013–2016 (HR, 0.41; 95% CI, 0.29–0.58) whereas it remained similar in patients with a mechanical prosthesis ().
Table 2. Hazard ratios for 28-day postoperative mortality after first surgical aortic valve replacement in Finland in 2001–2016.
Trends in 4-year prognosis after SAVR
The HRs for four-year risk of cardiovascular events i.e. myocardial infarction or stroke, all-cause mortality, and intracranial bleeding are presented in , respectively. Overall, the multivariable-adjusted risk of cardiovascular events decreased by 17% from 2001–2004 to 2005–2008 (HR, 0.83; 95% CI, 0.70–0.99) and 26% from 2001–2004 to 2009–2012 (HR, 0.76; 95% CI, 0.64–0.91). When analyzed separately, this trend was significant only in biologic valve group (HR 0.79; 95% CI, 0.63–0.98) (). The crude four-year postoperative mortality after all the aortic valve replacement procedures increased from 2001–2004 to 2009–2012 (HR, 1.23; 95% CI, 1.07–1.42), but stayed constant in the adjusted models (HR, 0.92; 95% CI, 0.80–1.05, ). The multivariable-adjusted four-year risk of risk of intracranial bleeding did not change over the study period in either the biologic nor mechanical valve groups ().
Table 3. Hazard ratios for 4-year incidence of cardiovascular events after first surgical aortic valve replacement in Finland in 2001–2016.
Table 4. Hazard ratios for 4-year postoperative mortality after first aortic valve replacement in Finland in 2001–2016.
Table 5. Hazard ratios for 4-year incidence of intracranial bleedings (I61 + I62) after first surgical aortic valve replacement in Finland in 2001–2016.
Discussion
The aim of this study was to examine how aortic valve replacement surgery has evolved from 2001 through 2016 in a nationwide setting in Finland. During the 16-year study period, the number of SAVRs using bioprosthesis increased, whereas the use of mechanical prostheses decreased. However, the mean age of the patients remained unchanged in both valve groups. The proportion of operated women declined markedly, especially in the last four-year period. During the last two time periods (2009–2012 and 2013–2016), short-term mortality has decreased and long-term mortality has stayed constant despite the increase in comorbidities. Risk of cardiovascular events after SAVR procedures has decreased, especially in the biologic valve group. Moreover, the proportion of concomitant CABG procedures diminished over the study period and the same trend was seen with both mechanical and biological valves.
The study by Martinsson et al. showed that the incidence of aortic stenosis does not differ markedly between men and women in the general population [Citation4]. Hence, our continually decreasing proportion of women undergoing SAVR is an interesting finding. This may root from various causes including presumed—and to some extent—a perceived higher risk of complications and mortality after open-heart surgery among women compared to men [Citation14]. As the survival of women after transcatheter aortic valve implantation seems to be better compared to men, this might lead physicians to refer women more easily either to TAVR or conservative treatment. Women are also typically older at the time of symptomatic aortic stenosis, which might also favor TAVR over surgery in women. The data on the gender distribution of TAVR patients in Finland was not available in our register. However, the nationwide FinnValve registry includes data from all TAVR-operations from 2008 to 2017 [Citation15]. Makikallio et al. discovered that the amount of TAVR-operations increased during the whole follow-up period, also TAVR patients were on average 81.2 years old, 55% were women and EuroSCORE II was on average 7.2% [Citation15]. Thus, decline in the proportion of women in 2013–2016 is probably related to increasing use of TAVR.
In the current European guidelines, bioprosthetic valves are recommended in patients aged older than 65 years and mechanical valves for patients aged under 60 years [Citation1]. In the American guidelines, however, bioprosthetic valves are recommended in patients aged older than 70 years and mechanical prosthesis in patients aged younger than 50 years, and either type in patients aged from 50 to 70 years [Citation8]. The mean ages of mechanical and biologic SAVR groups were 59.5 and 74.7 years, respectively, staying constant in both groups between 2001 and 2016. This reflects the guideline-based prosthesis selection and their implementation into practice [Citation1,Citation8]. The observed decrease in the number of mechanical valve procedures could partly be explained by overall higher age of the patients at the time of the surgery. This is also supported by a Swedish nationwide study which demonstrated that the median age at the time of the diagnosis of aortic stenosis has increased [Citation4]. It was discussed that the increasing use of lipid-modifying medications, angiotensin receptor blockers, and angiotensin converting enzyme inhibitors might indirectly slow the progression of aortic stenosis, although evidence on the effectiveness of medical management for the progression of aortic stenosis is at best weak.
Generally, the use of biological valves has increased [Citation9,Citation16], as also seen in our study. In our study population, however, the mean age of patients undergoing mechanical and biologic valve procedures stayed constant. The optimal cutoff age on the choice between mechanical and biological valve selection has been recently debated due to some studies with contradicting findings. A few studies support the use of biological valves in patients aged 50–69 years after finding no difference in mortality [Citation10,Citation17,Citation18]. Whereas other studies demonstrated lower long-term mortality in patients aged 50–69 years undergoing SAVR with mechanical prosthesis, in line with the current recommendations [Citation7,Citation9,Citation19].
According to some recent studies, the use of biological valves has increased also in younger age groups [Citation20], in contrast to current guidelines and recommendations [Citation1,Citation8]. One explanation for this change could be the development of newer generation bioprosthetic valves which tend to show very good long-term structural integrity [Citation21,Citation22] making physicians less reticent to choose biological valves for younger patients.
The proportion of patients with previous myocardial infarction decreased throughout the study period although other comorbidities like diabetes, previous stroke and hypertension increased. This most likely reflects the changing burden of disease in the study population. i.e., the incidence of coronary heart disease and stroke in Western countries has been steadily declining while hypertension and diabetes are on the rise [Citation6,Citation23]. In our study population, concomitant CABG procedures increased markedly over the study period, probably due to constantly increasing percutaneous coronary interventions [Citation6].
We observed a statistically significant increase in the proportion of urgent procedures over the study period. However, the change is only seen from 2001–2004 to 2005–2008. After 2005, the proportions of different urgency categories stayed rather constant. Before 2003, the information on urgency was not recorded directly in the registers and we therefore coded all pre-2003 procedures in which the patient had arrived in the hospital through the emergency room were defined as urgent. Taking this into account, it is possible that the increase in the urgent procedures is due to change in the reporting methods rather than a real change in clinical practice.
The strength of our study is the use of a comprehensive nationwide registry that includes virtually all patients who underwent SAVR in Finland over a period of 16 years. As reporting to the administrative registers used in our study is mandatory in Finland, our study is virtually free of selection bias. However, there are some limitations related to retrospective register data; the detail of the available data is limited and the register contained no information on the grading of the aortic valve disease. The New York Heart Association score could have been used to grade the severity of symptoms, but this was registered only for a small group of patients, and therefore excluded from the analyses. Moreover, reliable EuroSCORE data were unavailable because it has been included as part of the Finnish operative register starting in 2006 and our analyses extend back to 2001. Therefore, we included several key components of the EuroSCORE, such as age, sex, lung disease, urgency, and history of myocardial infarction, in our analyses.
This nationwide study demonstrated how patient selection and prognosis of SAVRs has changed over the past 16 years in Finland. The characteristics of patients have changed; the proportion of women decreased and the proportion of patients with coexisting conditions increased. Bioprosthetic valves became more common whereas the use of mechanical valves declined. At the same time, however, the patients are older at the time of surgery, which favors the use of biologic valves. Despite the increasingly morbid patient population, the short-term mortality improved and the long-term mortality remained unchanged.
Disclosure statement
Veikko Salomaa has participated in a conference trip sponsored by Novo Nordisk and received an honorarium from the same source for participating in an advisory board meeting. He also has ongoing research collaboration with Bayer Ltd. Tuomas Kiviniemi has given lectures for Bayer, BMS-Pfizer, MSD, Astra Zeneca, Orion Pharma; and received research grants for investigator-initiated studies from the Finnish Medical Foundation, the State Research Fund (Turku University Hospital), the Finnish Foundation for Cardiovascular Research, Atricure Ltd., USA and Finnish Cardiac Society. Ville Kytö has received lecture fees from Bayer and Boehringer-Ingelheim, travel grant from MSD, Bayer, AstraZeneca, Pfizer, and honorarium from AstraZeneca. Jarmo Gunn has received an unrestricted academic research grant from Vifor Pharma Ltd., Switzerland.
Additional information
Funding
References
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011.
- Martinsson A, Li X, Andersson C, et al. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015;131(11):988–994.
- Myllykangas ME, Aittokallio JM, Pietilä A, et al. Population trends in mitral valve surgery in Finland between 1997 and 2014: the Finnish CVD register. Scand Cardiovasc J. 2018;52(1):51–57.
- Kiviniemi TO, Pietila A, Gunn JM, et al. Trends in rates, patient selection and prognosis of coronary revascularisations in Finland between 1994 and 2013: the CVDR. EuroIntervention 2016;12(9):1117–1125.
- Glaser N, Jackson V, Holzmann MJ, et al. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years. Eur Heart J. 2016;37(34):2658–2667.
- Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2017;7(2):252.
- Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med. 2017;377(19):1847–1857.
- Chiang YP, Chikwe J, Moskowitz AJ, et al. Survival and long-term outcomes following bioprosthetic vs. mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA 2014;312(13):1323–1329.
- Mahonen M, Jula A, Harald K, et al. The validity of heart failure diagnoses obtained from administrative registers. Eur J Prev Cardiol. 2013;20(2):254–259.
- Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev. Rehabil. 2005;12(2):132–137.
- Tolonen H, Salomaa V, Torppa J, et al. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil. 2007;14(3):380–385.
- Wong SC, Yeo I, Bergman G, et al. The influence of gender on in-hospital clinical outcome following isolated mitral or aortic heart valve surgery. Cardiovasc Revasc Med. 2019;20(6):468–474.
- Makikallio T, Jalava MP, Husso A, et al. Ten-year experience with transcatheter and surgical aortic valve replacement in Finland. Ann Med. 2019;51:270–279.
- Glaser N, Sartipy U. Aortic valve replacement in middle-aged patients: is the increased use of bioprostheses justified? Expert Rev Cardiovasc Ther. 2016;14(4):405–406.
- Chikwe J, Chiang YP, Egorova NN, et al. Survival and outcomes following bioprosthetic vs. mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA. 2015;313(14):1435–1442.
- McClure RS, McGurk S, Cevasco M, et al. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J Thorac Cardiovasc Surg. 2014;148(5):1931–1939.
- Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg. 2008;135(4):878–884.
- Barnett SD, Ad N. Surgery for aortic and mitral valve disease in the United States: a trend of change in surgical practice between 1998 and 2005. J Thorac Cardiovasc Surg. 2009;137(6):1422–1429.
- Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837.
- Une D, Ruel M, David TE. Twenty-year durability of the aortic Hancock II bioprosthesis in young patients: is it durable enough? Eur J Cardiothorac Surg. 2014;46(5):825–830.
- Salomaa V, Pietilä A, Peltonen M, et al. Changes in CVD incidence and mortality rates, and life expectancy: North Karelia and National. Global Heart. 2016;11(2):201.
