Abstract
Introduction. There is limited knowledge about factors associated with the development of aortic stenosis. This study aimed to examine the prevalence of aortic sclerosis or stenosis in 71-years-old men and determine which risk factors at 50 years of age predict the development of aortic sclerosis or aortic stenosis. Methods. A random sample of Swedish men from the general population, born in 1943 (n = 798) were followed for 21 years. Data on clinical characteristics and laboratory values were collected in 1993. An echocardiography was performed in 2014. We used logistic regression to examine the association between baseline data and the outcome. Results. Echocardiography was performed in 535 men, and aortic sclerosis or aortic stenosis was diagnosed in 27 (5.0%). 14 persons developed aortic stenosis (2.6%). Among men with aortic sclerosis or aortic stenosis, 29.6% were obese. In multivariable stepwise regression model, body mass index (odds ratio per unit increase 1.23 (95% CI 1.10–1.38; p = .0003)) and hypercholesterolemia, combined with high sensitive C-reactive protein (odds ratio versus all other 2.66 (1.18–6.00; p = .019)) were significantly associated with increased risk of developing aortic sclerosis or aortic stenosis. Body mass index was the only factor significantly associated with a higher risk of developing aortic stenosis. Conclusion. The prevalence of either aortic sclerosis or aortic stenosis was 5% and of aortic stenosis 2.6%. Obesity and hypercholesterolemia combined with elevated high sensitive C-reactive protein at the age of 50 predicted the development of degenerative aortic sclerosis or stenosis, whilst only obesity was correlated with the occurrence of aortic stenosis.
Introduction
The association between conventional cardiovascular risk factors and the development of aortic stenosis (AS) in the general population remains insufficiently addressed, despite steeply increasing risk to develop AS with age [Citation1–3]. Recently, Yan et al. showed that hypertension, diabetes and hyperlipidemia have an independent association with AS in an unselected population of older individuals [Citation4]. However, in that study only severe AS was studied, defined as a combination of hospitalization and/or intervention for AS, without the use of echocardiographic data.
Contrary to the traditional view describing degenerative AS as a passive, ‘wear and tear’ process that results from normal aging, it is becoming apparent that an inflammatory process, at least partially triggered by cardiovascular risk factors, may also play an important role in AS pathophysiology [Citation5–7]. Since AS is the most common valvular heart disease that leads to intervention in developed countries [Citation8], understanding the pathophysiology of AS is essential, especially if there are modifiable risk factors associated with the development of AS. The primary aim of this study was, first, to determine the prevalence of echocardiographic-verified AS and aortic sclerosis in patients aged 71 years in a randomly selected cohort of Swedish men followed from age 50 to age 71. The second aim was to identify which cardiovascular factors (at 50 years of age) that predict the presence of AS or aortic sclerosis 21 years later.
Methods
Study population
The “Study of Men Born in 1943” is a longitudinal, prospective population study of middle-aged men. In 1993, a random sample of half of all men born in 1943 and living in the city of Gothenburg in Sweden was invited to participate in the study. Of the initial sample, 798 (54.5%) agreed to participate in a medical examination. All study participants were invited to follow-up examination in 2014. The population was mainly Caucasian and 82% of all participants were born in Sweden. Written informed consent was obtained from all participants. The study complies with the Declaration of Helsinki, and the study protocol was approved by the Ethical Committee of Gothenburg (DNR 157-93, 0067-03 and DNR 649-13) and registered in ClinicalTrials.gov (identifier: NCT03138122).
Examination at baseline
In 1993, a clinical examination was performed between 9 February 1993 and 21 June 1994. Body weight was measured to the nearest 0.1 kg with the participants wearing indoor clothing; height was recorded to the nearest cm while standing barefoot; body mass index (BMI) was calculated in the standard way, measured in kg/m2. Overweight was defined as BMI between 25 kg/m2 and <30 kg/m2 and obesity as BMI ≥30 kg/m2. Waist circumference was measured at the level of the umbilicus with the participant standing in an upright position. Blood pressure was measured by auscultation using a standard cuff and mercury manometer, with the participant in the sitting position. No correction was applied for arm circumference. Heart auscultation was a part of the physical examination. Hypertension was diagnosed based on either medical history with current anti-hypertensive therapy and/or current blood pressure ≥140 mm Hg(systolic) or 90 mmHg (diastolic).
Data on smoking habits, leisure time physical activity and previous diseases (including hypertension and diabetes) as well as pharmacological treatments were collected by questionnaires. Men who were current smokers or had quit smoking <1 month before the examination were categorized as smokers. Former smokers were defined as those who quit smoking >1 month before the examination and never smokers were defined as those who had never smoked cigarettes, cigars or a smoking pipe on a regular basis. Leisure time physical activity was assessed by the Saltin-Grimby Physical Activity Level Scale [Citation9] and coded as 1 = sedentary (almost completely inactive), 2 = some light physical activity (e.g. walking, riding a bicycle, light gardening) during at least 4 h per week, 3 = regular activity (e.g. running, heavy gardening, calisthenics) for a minimum of 3 h per week and 4 = regular hard physical training for competition (e.g. running events, racing).
Blood samples were drawn from an antecubital vein. Fasting serum cholesterol, serum triglyceride, serum glucose and serum calcium were determined according to standard laboratory procedures. Hypercholesterolemia was defined as either serum cholesterol >6.2 mmol/L or ongoing treatment with lipid-lowering medication [Citation10]. Diabetes Mellitus was defined as being present if positive medical history of diabetes mellitus, ongoing treatment with antidiabetics (insulin or oral) or fasting glucose >7 mmol/L at screening examination, according to World Health Association definition [Citation11]. Blood samples were also immediately centrifuged and stored in aliquots of serum –70 °C pending analysis in 2014 of high sensitive C-reactive protein (hs-CRP), high sensitive troponin T, hsTNT), and N-terminal pro brain natriuretic peptide (NT-proBNP).
Echocardiography
In 2014, all study participants underwent a standard echocardiography using a commercially available ultrasound scanner (Vivid 7, General Electric, Horten, Norway). The images were recorded from the parasternal long- and short-axis view, apical view of four-, two-, three- and five-chamber, subcostal and suprasternal view sweeps. The aortic valve was morphologically evaluated in the parasternal long- and short-axis view as well as apical views. The left ventricular outflow tract (LVOT) was measured in the parasternal long-axis view and the velocity over the LVOT and aortic valve was recorded in the apical five- or three-chamber view, whichever resulted in the highest velocities, using the 2 D transducer to obtain the best alignment with blood flow. In the case of chronic atrial fibrillation, the velocities were averaged over five beats. AS was defined as an anterograde velocity of ≥2.5 m/s recorded with continuous wave Doppler across the aortic valve and with thickened leaflets with reduced systolic opening on two-dimensional (2 D) imaging, according to the recommendations in use [Citation12]. Aortic sclerosis was defined as anterograde velocity across aortic valve between 2 m/s and 2.49 m/s, associated with thickened leaflets.
AS was classified according to the anterograde maximal velocity as mild (2.5–2.9 m/s), moderate (3–3.9 m/s) and severe (≥4 m/s). The patients who were operated with aortic valve replacement for AS during the 21-year follow up time were included in the severe AS category.
In those who died or who were not examined with echocardiography in 2014, their medical journals and their hospital discharge diagnosis codes were reviewed in order to collect data about known AS or aortic valve replacement for AS during the follow up period.
Statistical methods
Data analysis was generated using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR) as appropriate. Categorical variables were expressed as frequencies and percentages.
The univariable and multivariable associations between the baseline variables and the two subgroups of individuals, with aortic sclerosis or AS and without aortic sclerosis or AS, were determined by logistic regression. Variables with a possible association with the outcome were included in the analysis based on a clinical judgment. The best set of independent (explanatory) variables was chosen using stepwise selection. The effect size was described as a comparative index (odds ratios, ORs) with 95% confidence intervals (95%CI) and goodness-of-fit by area under the receiver-operating curve (ROC) with its 95%CI.
A subgroup analysis of the patients strictly with AS was performed. Taking into consideration the low outcome prevalence in this subgroup the Firths’s logistic regression method was used, with the same results. All tests were two-tailed and conducted at 0.05 significance level.
Results
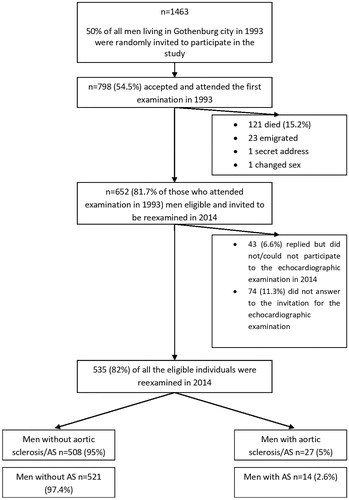
Out of the 798 men who participated in the initial examination, 677 (85%) were still alive in 2014. Of those men, 536 (79%) accepted to participate in the re-examination in 2014. As one man who initially accepted the invitation to the examination with echocardiography declined this once he was clinically examined, the final sample included 535 men. (). Among the 121 men who died between 1993 and 2014, four men (3.3%) had AS according to medical records; these individuals were not included in the final analysis.
Among the 535 men examined with echocardiography in 2014, 27 were diagnosed with aortic sclerosis or AS, yielding a prevalence of 5.0%. Among them, 14 had AS, resulting in a prevalence of AS of 2.6%. Of the 14 men with AS, five (36%) had mild, three (21%) moderate and six (43%) severe AS.
Of all men with aortic sclerosis or AS, 29.6% were obese at baseline, compared to 9.3% among men without aortic sclerosis or AS, 51.9% had hypercholesterolemia (versus 32.1%) and 7.4% had diabetes mellitus (versus 1.2%). There was no difference between men with and without aortic sclerosis or AS with respect to smoking habits and physical activity at baseline. Furthermore, no difference was found in occurrence of hypertension, levels NT-proBNP, hs-TnT, calcium levels and hs-CRP ().
Table 1. Demographic factors, risk factors and laboratory analysis among men with and without aortic sclerosis or AS.
In the strictly defined group with AS, 28.6% of the men with AS had a baseline BMI ≥30 kg/m2 vs. 9.8% in those without AS. There was no difference between men with and without AS in smoking habits, physical activity, hypercholesterolemia, or hypertension, NT-proBNP levels, diabetes, hs-TnT, calcium levels and hs-CRP at baseline (data not shown).
In univariable analysis, baseline BMI, both as a continuous and as a categorical variable (obesity-BMI ≥30 kg/m2), was significantly associated with the presence of either aortic sclerosis or AS, with an OR (95%CI) of 1.23 (1.10–1.38), p = .0002, and an OR of 4.13 (1.71–9.95), p = .0016, respectively (). The same result was obtained in the subgroup analysis with/without AS, where BMI, both as a continuous and as a categorical variable was significantly associated with an increased risk to develop AS. The OR was 1.18 (95%CI 1.02–1.37) per unit increase, p = .027, with BMI as a continuous variable whilst OR was 3.69 (95%CI 1.12–12.18), p = .032, with BMI as a categorical variable (<30 kg/m2 and ≥30 kg/m2). (, .
Figure 2. (a) Forest plot of the factors that predicted either aortic sclerosis or AS in univariable and multivariable analysis. (b) Forest plot of potential factors that predict AS in univariable analysis.
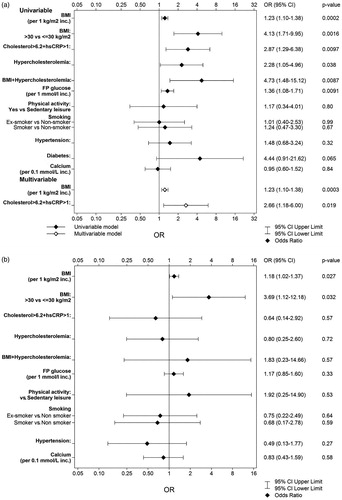
Table 2. Prediction analyses of aorta sclerosis or AS in 2014 by using logistic regression.
Table 3. Prediction analysis of aorta stenosis in 2014 using logistic regression.
Hypercholesterolemia at baseline was also a risk factor for developing either aortic sclerosis or AS 21 years later, with an OR (95%CI) of 2.28 (1.05–4.96), p = .038 (univariable analysis).
Obesity in combination with hypercholesterolemia had a stronger prediction for aortic sclerosis/AS than hypercholesterolemia alone: OR (95%CI) of 4.73 (1.48–15.12), p = .0087.
In a multivariable stepwise regression model performed on the groups with/without aortic sclerosis or AS, BMI as a continuous variable increased the risk of developing AS with an OR per unit of 1.23 (95%CI 1.10–1.38; p = .0003). Combining total cholesterol >6.2 mmol/l and hs-CRP >1 yielded an OR (95%CI) of 2.66 (1.18–6.00), p = .019, to predict the development of either aortic sclerosis or AS 21 years later (.
Discussion
Our study showed that the prevalence of aortic stenosis in Swedish men aged 71 is 2.6%, while the prevalence of either aortic sclerosis or AS is 5%. Overweight and obesity were associated with increased risk for either aortic sclerosis or AS after 21 years follow-up, while only obesity was associated with the presence of AS 21 years later.
To date, estimates of the prevalence of AS in the general population vary considerably, due to a) a lack of a common definition of AS, b) the application of different echocardiographic criteria, c) the use of cohorts that differ in characteristics in significant ways, d) different study design elements and e) the variable intensity and coverage of primary cardiovascular prevention in the different time periods in which these studies are conducted. Consequently, studies are, as a rule, not directly comparable. In some population studies with participants approximately 75 years of age [Citation13–16], the prevalence of AS has been between 2.4–5%. Obviously, the definition of AS is very important when the prevalence of AS is examined. Although it is now generally accepted that AS is present when the maximal velocity across the aortic valve is ≥2.5 m/s [Citation12], other guidelines defined mild AS as maximal transvalvular velocity between 2–2.9 m/s [Citation17]. Some of the prevalence studies used this broader definition of AS, partly explaining the different results in the prevalence of AS. When applying these two definitions for AS, the prevalence in our study was 2.6% with the stricter definition and 5% with the broader definition that included aortic sclerosis. Our finding is in line with one study that used the same AS definition (i.e. peak aortic flow velocity ≥2.5 m/s) and reported a prevalence of 2.4% in patients aged 65 to 74 years [Citation15]. However, the prevalence of AS in our study is lower than in The Helsinki Ageing Study, performed in older patients aged 75–86 years, in which at least 4.8% of participants had at least moderate AS [Citation14]. Likewise, in the Tromsö study in which AS was defined as a transvalvular mean gradient of ≥15 mmHg the reported prevalence was 3.9% in the 70–79-year-old group and 9.8% in the 80–89-year-old group [Citation16].
Cumulative evidence supports the view that degenerative calcific aortic valve disease represents a chronic, progressive, proliferative and inflammatory process with risk factors similar to those associated with atherosclerotic vascular disease [Citation4,Citation18–21]. It is thus conceivable that the modification of such risk factor might reduce the incidence of AS. Recently, Yan et al demonstrated that hypertension, diabetes, and dyslipidemia have independent and dose-response associations with incident AS in an unselected population of older individuals, and together accounted for approximately one-third of the incidence of severe AS [Citation4]. However, the endpoint in this study was the incidence of severe AS that required hospitalization or aortic valve intervention rather than echocardiography verified AS or aortic sclerosis, which is the definition used in our study.
Furthermore, in our study overweight and obesity, as well as hypercholesterolemia in combination with inflammation at 50 years of age were associated with more prevalent aortic stenosis/sclerosis during two decades´ of follow-up. Available data addressing associations between conventional risk factors and risk of developing AS are not consistent. Some showed positive associations between obesity and aortic valve calcification or AS [Citation20,Citation22–26], whereas others did not [Citation27]. Similarly, some showed positive associations between AS and hypercholesterolemia [Citation21–23] or dyslipidemia [Citation4] whereas other did not [Citation27]. In our study, we assessed all conventional risk factors at baseline: hypertension, diabetes, BMI >30, hypercholesterolemia, smoking, sedentary lifestyle. Among them, overweight and obesity as well as hypercholesterolemia in combination with inflammation at 50 years of age were associated with increased risk for aortic stenosis after 21 years. We combined hypercholesterolemia with hs-CRP because of contradictive results from the studies concerning the correlation between AS and hypercholesterolemia. As the incipient lesions in AS and those of atherosclerosis share some similar features [Citation5–7], we considered that the combination of both high cholesterol and a low-grade of inflammation might be additive in predicting AS. Our data might provide some support for the hypothesis that the development of AS shares some risk factors with atherosclerosis, such as high BMI and hypercholesterolemia, but seems only when it is associated with a grade of inflammation.
The association between AS and hypercholesterolemia has also been demonstrated in animal studies [Citation28–31], but the benefit in the prevention of AS by treating hypercholesterolemia could not be demonstrated in humans in randomized studies [Citation32–35]. Nevertheless, some smaller studies suggested that there might be a positive effect of statin treatment in delaying AS development, with the effect apparently independent of cholesterol levels [Citation36–40]. This leads to the possibility to speculate that the intervention with statins should be undertaken early in the development of the valvular lesion, since the treatment was proved inefficient in a later phase in randomized trials [Citation32–35,Citation41]. Furthermore, in our study, hs-CRP in combination with hypercholesterolemia reached statistically significant level in the prediction of AS after 21 years. Our result strengthen the hypothesis that inflammation might be a part of the complex underlying mechanism behind development of AS [Citation42–44], having a synergistic effect with hypercholesterolemia.
Our findings might provide a new insight into the mechanism for developing AS. Based on our results we suggest that in order to prevent or slow down development of aortic sclerosis and its progression to AS, preventive measure should not only be applied at an early stage of the disease, but also should focus on multiple risk modifications (overweight, higher BMI, hypercholesterolemia and inflammation).
Being so closely correlated with atherosclerosis, one may intuitively consider diabetes as a predictive factor for AS. However, we could not find a significant correlation between diabetes at 50 years of age and risk of developing AS 21 years later. This might be because our study is a 21-year longitudinal observation and some individuals without diabetes at baseline might have developed diabetes later on, but also because there was a low event rate in our study, with few patients with AS. Thus, our results do not exclude the possibility that disturbed glucose metabolism might be involved in the development of AS as other studies have indicated [Citation4,Citation21,Citation22,Citation45].
In contrast with earlier studies [Citation4,Citation15,Citation22,Citation27,Citation45], we did not find a correlation between AS and hypertension, potentially because of better treatment of hypertension nowadays. Likewise, smoking failed to predict AS after 21 years in our study; this could be partly be explained by selective survival in non-smokers, resulting in fewer smokers alive to be diagnosed with AS in 2014. In contrast, another Swedish study found that current smoking is associated with aortic stenosis [Citation46]. In addition, successful strategies used in order to decrease smoking in the population has reduced the number of smokers in industrialized countries [Citation47].
Our data indicates the importance of new longitudinal studies in the era of successful implementation of cardiovascular prevention strategies, because, as a result, conventional risk factors that are implicated have changed. It has recently been shown that the incidence and mortality rates of AS declined lately, paralleling those of myocardial infarction and heart failure [Citation48], suggesting that intervening on risk factors might be a key factor in lowering the burden of AS.
Strengths and limitations
The 21-year follow-up design of a middle-aged random population sample and echocardiography-verified aortic sclerosis and AS as a composite outcome are major strengths of this study, as aortic sclerosis might reasonably be regarding as an incipient stage of AS. Our longitudinal follow-up makes possible to assess the cumulative risk of progression of aortic sclerosis to AS. Moreover, all participants were of the same age, sex, and living in the same geographical area, making possible to study a rather homogenous sample, eliminating certain confounding factors.
The major limitation of our study is the low event rate, creating uncertainty in the interpretation of our results. A second limitation is that our cohort is representative of developed, western countries, most individuals were of Caucasian decent and our results cannot therefore be extrapolated to populations of other origin or living in developing countries. A third limitation is that we did not perform an echocardiographic examination at baseline. Heart auscultation could have excluded most cases of AS at baseline, which would, likely have become clinically manifest over the 21-year follow-up. Thus, being aware that the presence of mild aortic sclerosis or aortic stenosis at baseline cannot be completely excluded clinically, in our study we are only able to report data on prevalence at age 71, irrespective of at what age the development of AS started. A fourth limitation is that only men were included in our study. The prevalence of aortic stenosis was shown to be twice as high in men as in women [Citation15]. Therefore, the prevalence that we found could be overestimated if applied to the whole population of both genders. Furthermore, we did not have data on how many patients had bicuspid aortic valve. Finally, some individuals who died or were not examined by echocardiography in 2014 might have had undiagnosed AS, thus death being a competing event in these cases.
Conclusion
We found aortic sclerosis or AS stenosis in 5% of a random population sample of men, aged 71 years while strictly defined AS occurred in 2.6%. Our findings underline the importance of early intervention over the conventional risk factors, especially overweight and hypercholesterolemia combined with inflammation in an attempt to slow down or prevent the development of AS.
Authors contributions
Silvana Kontogeorgos and Erik Thunström participated in analysis and interpretation of data, in drafting the manuscript. Carmen Basic, You Zhong, Constantinos Ergatoudes, David Morales, Zacharias Mandalenakis were involved in the data acquisition, data interpretation, and revising the manuscript. Per-Olof Hansson, Annika Rosengren, Kenneth Caidahl and Michael Fu designed the study, and were of major importance in data interpretation and in revising critically the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy
Disclosure statement
Silvana Kontogeorgos, Carmen Basic, Per-Olof Hansson, You Zhong, Constantinos Ergatoudes, David Morales, Zacharias Mandalenakis, Annika Rosengren, Kenneth Caidahl, declare no conflict of interests.
Erik Thunström MD PhD have received consultation/lecture fees from Pfizer och Resmed
Michael Fu received consultation/lectures fees from AstraZeneca, Boehringer Ingelheim, Novartis, Resmed, Servier, and ViforPharma.
Additional information
Funding
References
- Otto CM, Prendergast B. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N Engl J Med.. 2014;371(8):744–756. 21
- Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438–2488.
- Vahanian A, Alfieri O, Andreotti F, et al. [Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)]. G Ital Cardiol. 2013;14(3):167–214.
- Yan AT, Koh M, Chan KK, et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. 2017;69(12):1523–1532.
- Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853.
- O'Brien KD, Reichenbach DD, Marcovina SM, et al. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16(4):523–532.
- Gould ST, Srigunapalan S, Simmons CA, et al. Hemodynamic and cellular response feedback in calcific aortic valve disease [celular biology….]. Circ Res. 2013;113(2):186–197.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24(13):1231–1243.
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38(6):1104–1115.
- Fu M, Rosengren A, Thunstrom E, et al. Although coronary mortality has decreased, rates of cardiovascular disease remain high: 21 years of follow-up comparing cohorts of men born in 1913 with men born in 1943. JAHA. 2018;7(9):e008769
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197.
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1–23.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011.
- Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21(5):1220–1225.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634.
- Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis. the Tromso study [epidemiology- important]. Heart. 2013;99(6):396–400.
- Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148(1):e1–e132.
- Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113(17):2113–2119.
- Cao J, Steffen BT, Budoff M, et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol.. 2016;36(5):1003–1009.
- Owens DS, Katz R, Takasu J, et al. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am J Cardiol. 2010;105(5):701–708.
- Deutscher S, Rockette HE, Krishnaswami V. Diabetes and hypercholesterolemia among patients with calcific aortic stenosis. J Chronic Dis. 1984;37(5):407–415.
- Messika-Zeitoun D, Bielak LF, Peyser PA, et al. Aortic valve calcification: determinants and progression in the population. ATVB. 2007;27(3):642–648.
- Thanassoulis G, Massaro JM, Cury R, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol. 2010;55(22):2491–2498.
- Larsson SC, Wolk A, Hakansson N, et al. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur Heart J. 2017;38(28):2192–2197.
- Peltier M, Trojette F, Sarano ME, et al. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol. 2003;91(1):97–99.
- Larsson SC, Back M, Rees JMB, et al. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2019:pii: 5514076. ehz388, 10.1093/eurheartj/ehz388.
- Lindroos M, Kupari M, Valvanne J, et al. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15(7):865–870.
- Weiss RM, Ohashi M, Miller JD, et al. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114(19):2065–2069.
- Miller JD, Weiss RM, Serrano KM, et al. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119(20):2693–2701.
- Zahor Z, Czabanova V. Experimental atherosclerosis of the heart valves in rats following a long-term atherogenic regimen. Atherosclerosis. 1977;27(1):49–57.
- Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res. 2013;113(2):209–222.
- Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–2397.
- Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356.
- Teo KK, Corsi DJ, Tam JW, et al. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27(6):800–808.
- Chan KL, Teo K, Dumesnil JG, et al. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121(2):306–314.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49(5):554–561.
- Novaro GM, Tiong IY, Pearce GL, et al. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104(18):2205–2209.
- Bellamy MF, Pellikka PA, Klarich KW, et al. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40(10):1723–1730.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110(10):1291–1295.
- Aronow WS, Ahn C, Kronzon I, et al. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88(6):693–695.
- Dichtl W, Alber HF, Feuchtner GM, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol. 2008;102(6):743–748.
- Fox CS, Guo CY, Larson MG, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol. 2006;97(10):1502–1505.
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60(19):1854–1863.
- Nagy E, Andersson DC, Caidahl K, et al. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123(12):1316–1325.
- Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59(9):998–999.
- Larsson SC, Wolk A, Back M. Alcohol consumption, cigarette smoking and incidence of aortic valve stenosis. J Intern Med. 2017;282(4):332–339.
- Thun M, Peto R, Boreham J, et al. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21(2):96–101.
- Martinsson A, Li X, Andersson C, et al. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015;131(11):988–994.