Abstract
Objectives. The present study aimed to evaluate prognostic value of inflammatory markers for in-hospital mortality and renal complication in patients undergoing surgery for acute type A aortic dissection (ATAAD). Design. Serum concentration of C-reactive protein, leukocyte counts, procalcitonin (PCT), tumor necrosis factor (TNF)-α, interleukin-2 receptor (IL-2R), IL-6 and IL-8 were measured on the day of admission to the hospital (T0) and on 1st (T1), 2nd (T2), and 7th (T3) day after surgery. Results. 328 patients were included. There were significant differences between survivor group and non-survivor group in PCT, IL-2R, and IL-6 (p = .001, p = .015, and p = .005). There were significant differences between patients with different AKI stage in PCT and IL-2R (p = .001, p < .001). The area under receiver operating characteristics (ROC) curve on 30-day death was 0.686 for PCT, 0.718 for IL-2R, 0.694 for IL-6 and 0.627 for IL-8. The area under ROC curve on stage III AKI was 0.852 for PCT, 0.749 for IL-2R, 0.626 for IL-8, and 0.636 for TNF-α. IL-2 > 1438 U/ml and IL-6 > 45.5 pg/ml were independently associated with 30-day mortality (p = .014 and p < .001). The area under ROC curve was 0.849 on score 2 (using 1 point for PCT > 4.58 ng/ml, 1 point for IL-2R > 1438 U/ml, 1 point for APACHE II score >15.5, and 1 point for IL-6 > 45.5 pg/ml). Conclusions. PCT and cytokines may be considered as predictors for adverse renal outcomes and mortality in patients with ATAAD patients after surgery. They are earlier than traditional biomarkers and combination of these biomarkers will improve the accuracy.
Introduction
Acute type A aortic dissection (ATAAD) remains a life-threatening disease, with mortality of estimated at 1–2% increase per hour if unrepaired. Recent data from the International Registry of Acute Aortic Dissection (IRAD) show a decrease in operative mortality from 31 to 22% over a 17-year time period [Citation1], which associated with the increasing number of operations performed per year [Citation2]. Preoperative predictive risk factors for in-hospital mortality after repair of ATAAD involve renal failure, aged patient, shock, cardiopulmonary resuscitation, remote myocardial infarction, and indicators of systemic malperfusion [Citation3,Citation4]. Intraoperative and postoperative predictors of death after ATAAD repair include the need for renal replacement, cardiopulmonary bypass (CPB) and clamping time, excessive bleeding, and arch replacement [Citation5,Citation6]. Acute kidney injury (AKI) is a common complication and typically occurs in 44% of patients with ATAAD after operation [Citation7]. AKI is an independent risk factor for mortality, with consequently extended intensive care unit (ICU) stay, hospital stay, leading to increase the need for short-and long-term renal replacement therapy, as well as progression of chronic kidney disease [Citation8]. Large body mass index, sex, and prolonged duration of CPB were identified as independent predictors for postoperative AKI [Citation9].
The underlying mechanisms of postoperative complications and the cause of death remain elusive. Systemic inflammatory reactions play a vital role after the onset and in development of ATAAD [Citation10]. Ascending aorta replacement and stent implantation along with surgery trauma and CPB may aggravate this response. Inflammatory responses can contribute to capillary leakage and the development of postoperative complications, including ventricular dysfunction, respiratory failure, renal and liver dysfunction, neurological dysfunction, bleeding, and eventually multiple organ failure.
The extent of cytokine secretion and systemic inflammatory response has been enlisted to be useful for stratifying the risk of surgical and critically ill patients [Citation11]. Cytokines and other inflammatory mediators may contribute to the pathogenesis of post-surgical complications and become biomarkers for continuous renal replacement therapy (CRRT) [Citation12]. Monitoring the levels of these biomarkers may be useful in early diagnosis, guiding CRRT, measuring the progress, and predicting the prognosis in patients. However, at present, little information is available about the effects of perioperative inflammatory biomarkers on patients undergoing aortic dissection surgery. In this study, we hypothesized that persistent elevation of inflammation in early stage of postoperative acute aortic dissection maybe a predictor for adverse clinical outcome.
Materials and methods
Study cohort
This single-center retrospective study was performed between November 2014 to June 2017 in the Department of Cardiac Surgery Intensive Care Unit, Zhongshan Hospital, Shanghai Medical College, Fudan University (Shanghai, China). The study was approved by the Ethics Committee of Zhongshan Hospital (Approval Number: B2016-142R). The board waived the requirements for written consent from the patients.
All consecutive patients who were admitted to cardiac surgery ICU of our hospital and diagnosed as ATAAD (chest pain or other related symptoms, which presented less than 14 days before the operation) were included. Exclusion criteria were: (1) patients died due to aortic dissection rupture or choosing conservative treatment; (2) patients died within 24 hours after surgery.
During the study period, four cardiac surgeons were involved in surgical management. Emergency surgery is defined as admission to operation less than 48 h. All procedures involved a median sternotomy and total CPB. CPB was instituted using right axillary artery or femoral artery perfusion and right atrial venous drainage. Patients underwent aortic arch surgery with unilateral antegrade selective cerebral perfusion through the brachiocephalic artery and deep hypothermic circulatory arrest (DHCA). Core cooling was carried out with an esophageal temperature of 20–25 °C.
Sample collection and biomarker measurements
Serum samples were collected on the day of admission to the hospital (T0) and on 1st (T1), 2nd (T2), and 7th (T3) day after the surgery. Serum concentration of C-reactive protein (CRP), leukocyte counts, procalcitonin (PCT), tumor necrosis factor (TNF)-α, interleukin-2 receptor (IL-2R), IL-6, and IL-8 were measured. Venous blood samples (5–10 ml) were taken in vacutainer tubes under sterile conditions from patients. Serum was obtained from freshly drawn, rapidly centrifugated, quickly frozen at −70 °C and stored until processed. Serum concentration of IL-6 was analyzed on the Siemens IMMULITE 2000 system using an IMMULITE® 2000 IL6. Serum concentration of TNF-α, IL-2R, IL-8 were analyzed using an IMMULITE® 2000 IL2. We blinded the personnel who measuring the biomarkers to clinical outcomes, and samples were analyzed according to manufacturer specifications. Illness severity was assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores at T1. Duration of mechanical ventilation, ICU and hospital length of stay, and complications were recorded as well.
Outcome definitions
The outcome variable was in-hospital death within 30 days after operation. The other outcome variable was postoperative AKI according to the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI of 2012 [Citation13] within 7 days after operation. Stage I AKI is defined by an increase in serum creatinine by 1.5–1.9 times baseline or ≥26.5 μmol/L, Stage II AKI is defined by an increase in serum creatinine by 2.0–2.9 times baseline, and stage III AKI is defined by an increase in serum creatinine by ≥3.0 times baseline or increase in serum creatinine to ≥354μmol/L, or a new requirement for dialysis. Preoperative serum creatinine values that were the nearest to the time of surgery were used as baseline levels. We modified the original definition because data on urine output were inaccuracy when collected retrospectively.
Statistical analysis
Quantitative data were statistically expressed according to its distribution. Normally distributed continuous variables are presented as mean ± standard deviation (SD), while non-normally distributed continuous variables are presented as median (interquartile range [IQR]) and were logarithmically (Lg) transformed for further analyses. Qualitative variables were expressed as frequencies. Student’s t-test was used to evaluate differences between groups. Categorical variables were analyzed by Chi-Square test. A two-way, repeated measure ANOVA was used to compare the effects of time (T0, T1, T2, T3) and AKI/non-survival status for all variables. A Bonferroni correction was used for post hoc pairwise comparison of means. To explore the relationship between biomarkers and duration of CPB and aortic clamping, as well as clinical outcomes and scores, Pearson analysis was used. The optimum cutoff points were determined with a receiver operating characteristics (ROC) curve. We next examined the association between biomarker measurements and mortality, by using multivariable logistic regression. Those same cutoff values in ROC curve were used to dichotomize the variables for multivariate logistic regression analysis. We adjusted for age, sex and operative characteristics.
Finally, we analyzed the prognostic utility of combining several novel biomarkers together with APACHE II score, into a simple additive risk score for the mortality. A discrete score was calculated for each patient, with 1 point assigned for each biomarker and an APACHE II value above the cutoff values, creating a maximum possible score of 3 or 4 points. ROC curves were constructed to evaluate the discriminant utility of this combination risk score for the mortality. The Youden index was applied to set the cutoffs and compared between the combined evaluation method and single evaluation methods. Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when two-tailed p value <.05.
Results
Patient characteristics
Among 340 patients were enrolled in the present study, 10 patients were excluded because of aortic dissection rupture or choosing conservative treatment. After exclusion of two patients due to their death within 24 hours after operation, 328 patients were finally analyzed (). 243 (74%) patients were male and 85 (26%) cases were female. Patients’ age ranged from 20 to 75 years (51 ± 12 years). shows demographic data and characteristics of the patients. Mean pump-time, cross-clamp time and DHCA time were 183 ± 35, 107 ± 26, and 24 ± 8 min, respectively. Surgical procedures are listed in . Concomitant procedures included coronary artery bypass grafting in 15 patients, aortic-femoral artery bypass in 15 patients, mitral valve replacement/plasty in 4 patients and atrial septal defect repair in 1 patient. Of the 328 patients, 83 (25.3%) patients developed AKI stage I, 51 (15.5%) patients developed AKI stage II, and 105 (32%) patients developed AKI stage III. 31 (9.5%) patients died within 30 days after operation.
Table 1. Demographic and characteristics of patients with ATAAD.
Correlations between inflammatory markers and operative factors
There were correlations between PCT levels at T1 and duration of CPB (p < .001, r = 0.316). Same results were found between PCT levels and duration of aortic clamping (p = .002, r = 0.234). IL-2R levels at T1 also had correlations with duration of CPB (p = .013, r = 0.214), but had no correlations with duration of aortic clamping. However, there were no significant correlations between other markers (TNF, IL-6, and IL-8) and CPB or cross-clamp time. IL-2R at T1 had a significant correlation with red cell transfusion (r = 0.295, p < .001) and fresh frozen plasma (r = 0.247, p < .001) received by patients during operation. PCT at T1 also had a significant correlation with red cell (r = 0.228, p < .001) and fresh frozen plasma transfusion (r = 0.199, p = .001; ).
Table 2. Factors affecting inflammatory markers at T1.
Comparison of studied inflammatory markers between different groups
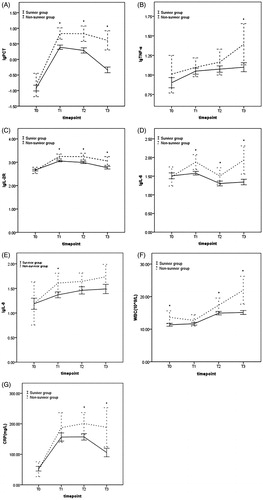
As illustrated in , the serum concentrations of PCT, IL-2R, and IL-6 were significantly higher in the non-survivor group on all postoperative days than in the survivor group. The levels of TNF-α only differed significantly between the groups at T3 (), and the IL-8 only differed at T1 (). WBC and CRP were higher in the non-survivor group at T2 (p = .036, p = .021) and T3 (p < .001, p = .003) compared with that in the survivor group.
Figure 2. Comparing levels of lgPCT, lgTNF-α, lgIL-2R, lgIL-6, lgIL-8, WBC, and CRP between survivor group and non-survivor group (*p < .05).
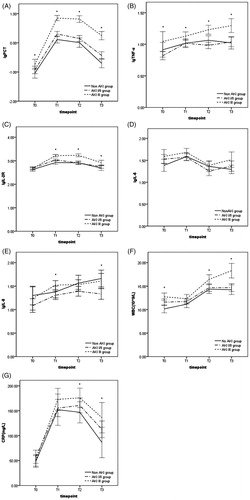
There were statistically significant differences between patients with and without AKI stage III in PCT and TNF-α from T0 to T3, and in IL-2R from T1 to T3 (). However, between AKI stage I/II and non-AKI group, only serum concentration of PCT and IL-2R at T1 differed significantly (p < .001). IL-6 did not have any significant differences between groups (). The levels of IL-8 in AKI I/II group were significantly lower than that in AKI stage III group at T1, meanwhile lower than that in AKI III and non-AKI group at T3 (). WBC was significantly higher in patients with AKI stage III than in those without AKI stage III at T0 (p < .001), T2 (p = .004), and T3 (p < .001). The level of CRP was higher in AKI stage III group than non-AKI group only at T3.
Relationship between inflammatory markers and clinical outcomes
The SOFA and APACHE II score systems can assess disease severity and predict clinical outcomes in critically ill patients. Serum concentrations of PCT, IL-6, IL-8, and IL-2R were significantly correlated with the SOFA score. Serum levels of PCT, IL-8, CRP, and IL-2R were significantly correlated with the APACHE II score (). In multivariate analysis, serum levels of IL-2R and IL-6 were correlated with the SOFA score independently, and serum levels of PCT and IL-2R were correlated with the APACHE II score independently.
Table 3. Correlations among SOFA and APACHE II scores and serum concentrations of studied biomarkers in the overall group of patients.
There was a correlation between serum concentration of PCT at T1 and the duration of mechanical ventilation (p < .001, r = 0.231), LOS in ICU (p < .001, r = 0.288). The level of IL-2R was correlated with duration of mechanical ventilation (p = .003, r = 0.209) and with LOS in ICU (p = .001, r = 0.237). The level of IL-8 was correlated with the duration of mechanical ventilation (p = .010, r = 0.181).
ROC curves of biomarkers for mortality and AKI stage III
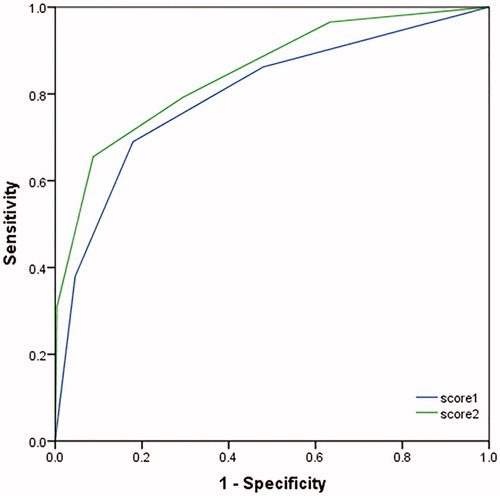
A relationship between biomarker concentrations and clinical outcomes was tested by ROC curve (). The area under the curve (AUC) (95% confidence interval) on 30-day death was 0.686 (0.555-0.817) for PCT, was 0.718 (0.598-0.817) for IL-2R, 0.694 (0.556-0.832) for IL-6, 0.627 (0.498-0.756) for IL-8, 0.786 (0.679-0.893) for SOFA, and 0.770 (0.652-0.889) for APACHE II. The cutoff values of those markers and scores are shown in . The AUC on AKI stage III was 0.852 (0.793-0.910) for PCT, 0.749 (0.670-0.827) for IL-2R, 0.588 (0.497-0.680) for IL-6, 0.626 (0.537-0.714) for IL-8, 0.636 (0.544-0.728) for TNF-α, 0.761 (0.688-0.835) for SOFA, and 0.772 (0.696-0.849) for APACHE II. The cutoff values of biomarkers and scores for AKI stage III are shown in . Novel inflammatory biomarkers demonstrated proper ability to predict the early diagnosis and development of AKI.
Figure 4. ROC curve of the relationship between concentrations of biomarkers at T1 and mortality (A) or AKI stage III (B).
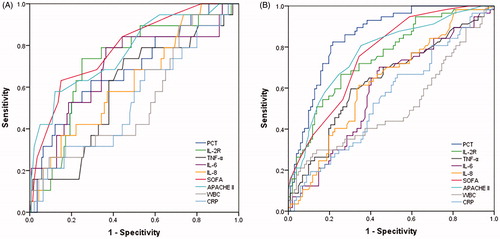
Table 4. Cutoff values of inflammatory markers, cytokines and scores to predict mortality.
Table 5. Cutoff values of inflammatory markers, cytokines, and scores to predict stage III AKI.
Combination of biomarkers to predict 30-day mortality
We used the measured parameters to define a categorical-based scoring system in an attempt to better prediction for mortality. We analyzed the score1 (using 1 point for PCT > 4.58 ng/ml, 1 point for IL-2R > 1438 U/ml and 1 point for APACHE II score >15.5) and score2 (using score1 plus 1 point for IL-6 > 45.5 pg/ml) of each patient on a ROC. The AUC was 0.798 (95% CI 0.703–0.892, p <0.001) on score 1 and 0.849 (95% CI 0.768–0.930, p < .001) on score 2, respectively (). The AUC values for the combined parameter score were more predictive than any one individual marker.
Multivariate analysis to predict 30-day mortality
Univariate analysis detected PCT, IL-2R, IL-6, IL-8, SOFA, and APACHE II scores at T1 demonstrating a significant association with the 30-day mortality. Multivariate logistic regression analysis showed that IL-2R > 1438 U/ml and IL-6 > 45.5 pg/ml were independently associated with 30-day mortality (OR 4.307, 95% CI 1.349–13.753, p = .014; OR 8.779, 95% CI 2.865–26.897, p <.001, respectively). After adjustment for CPB time and intraoperative red blood cell transfusion, age and IL-2R > 1438 U/ml remained independently associated with mortality (p = .017 and p = .009; ).
Table 6. Multivariate analysis of in-hospital mortality within 30 days.
Discussion
In this study, we evaluated the usefulness of postoperative inflammatory markers in predicting mortality and renal complication in ATAAD patients. The most important findings are presented as follows: (1) there is an obvious increase of those markers which reflecting inflammatory reaction in postoperative period and their persistent elevations are associated with renal complication and mortality; (2) compared to traditional inflammatory biomarkers such as WBC and CRP, novel markers such as PCT, IL-2R, and IL-6 were more accurate to predict mortality, PCT, IL-2R, and TNF-α were more accurate to predict AKI stage III; (3) the AUC values for the combined parameter score were more predictive than any individual marker. These novel markers may serve as biomarkers of adverse outcomes and further study of the inflammatory process may provide possible therapies to reduce morbidity and mortality in this population.
The perioperative release of inflammatory markers following cardiac surgery is affected by several factors and processes such as tissue damage, contact activation with non-endothelial surfaces, reperfusion injury, perioperative administration of drugs, hyperthermia during rewarming, haemodilution and the likelihood of endotoxemia. Aortic surgery along with greater surgical trauma, ischemia-reperfusion injury related to aortic clamping, local cellular interactions arising at the blood/biomaterial interface and massive transfusion has greater systemic inflammatory response than other cardiac surgery [Citation14]. The levels of PCT, IL-2R reached a peak at first day after operation and then gradually decreased. We also found that the peak was correlated with duration of CPB, red cell and fresh frozen plasma transfusion received by patients during operation. Transfused blood may have immunomodulatory and proinflammatory effects. Brocca et al. reported increased levels of PCT and IL-6 in patients with higher transfusion need [Citation15]. Jiwaji et al. expressed by a randomized controlled trial that leukoreduced blood transfusion does not increase circulating soluble markers of inflammation [Citation16]. However, serum concentrations of TNF-α, IL-8, and WBC showed an uptrend during the first week after operation. CRP had a peak at the second day after operation. All these biomarkers had no relationship with duration of CPB and the amount of infused blood.
Acute systemic inflammatory response is considered as one of the key factors associating with postoperative morbidity and mortality [Citation17]. Markers such as, cytokines, TNF-α, and PCT were demonstrated to be involved in the appearance of systemic inflammation. Simultaneously, a correlation between the magnitude of cytokine response and the severity of organ injury was found. TNF-α is a 17kD molecular, secreted by macrophages, mast cells, and natural killer cells, with the effect of stimulating the release of proinflammatory cytokines and prostaglandin inflammatory mediators from macrophages [Citation18]. IL-6 is a 26kD protein produced by various cells including the Kupfer cells, lymphocytes, activated macrophages, endothelial cells, adipocytes, and fibroblasts. It is considered to be a major mediator of the acute phase response to CPB and in case of thoracic aorta and valve surgery [Citation19]. IL-2 is a cytokine playing a critical role in the normal function of the immune system and is primarily produced by activated T lymphocytes. IL-2R, consisting of IL-2Ra (CD25), IL-2Rb (CD122), and γc (CD132), is primarily found on activated conventional T and regulatory T cells [Citation20]. IL-8 is a pro-inflammatory cytokine that play a definite role in the regulation of neutrophil recruitment and migration [Citation21]. These pro-inflammatory cytokines may contribute to additional organ dysfunction, which is associated with high mortality. PCT has previously been considered as a reliable marker for acute bacterial infection, while it holds tremendous promise to identify a diverse set of medical conditions and organ dysfunction [Citation22]. Our previous research demonstrated that PCT level and its change trend after Type A aortic dissection surgery might serve as early prognostic markers to predict postoperative outcomes [Citation23]. In the present study, survival patients showed lower serum levels of IL-2R, IL-6, IL-8, and PCT compared with non-survival patients. ROC curves and multivariate analysis also showed that PCT, IL-2R, and IL-6 demonstrated appropriate ability to predict mortality as well.
Some authors found that an enhanced score system, where common clinical biomarkers are combined with other clinical laboratory information, can provide a more reliable diagnosis and prediction tool for mortality [Citation11]. To enhance the diagnostic value of single parameter, we combined APACHE II and biomarkers to build a scoring system to improve diagnostic abilities. Our study found that the AUC of score2 (using 1 point for PCT > 4.58 ng/ml, 1 point for IL-2R > 1438 U/ml, 1 point for IL-6 > 45.5 pg/ml and 1 point for APACHE II score >15.5) at the first day after operation to predict mortality can reach 0.849. The score is easy to get and simple to follow up in daily clinical work. This method provided a better prediction of mortality than any of the other biomarkers in isolation that were assessed in this study.
The increased incidence of AKI is known as a common postoperative complication after aortic surgery [Citation24]. Nephrotoxins, hemodynamic instability, hypoxia, and inflammation all contribute to postoperative AKI. 31.9% of AKI stage III was observed in our study population, and there were statistically significant different levels of PCT, TNF-α, and IL-2R before and after operation between the patients with and without post-operative AKI stage III. Numerous studies have shown that levels of serum cytokines, such as TNF-α and IL-6, are increased in patients with severe AKI [Citation25]. IL-6 can be also upregulated and released from renal tubular epithelial cells in response to injury, playing a key role in the pathophysiology of AKI [Citation26]. However, we did not find any difference of IL-6 levels between the groups of AKI. In the absence of sepsis, PCT may be a proper marker for non-specific inflammation, leading to a greater inflammatory response and a higher risk of AKI [Citation27]. Previous research indicated that cytokines have a direct role in the pathogenesis of AKI and are elevated in AKI states. There is also an evidence that cytokines are cleared by the kidney and elevated when there is a decreased of renal function [Citation28]. However, the degree and the type of elevated cytokine levels are causing AKI or simply surrogate markers of decreased renal clearance has not yet been determined. Otherwise, compared to the good ability to predict AKI stage III, there were not such excellent performance of these biomarkers to predict AKI stage I/II. Only the level of PCT and IL-2R at the first postoperative day differed between AKI stage I/II and non-AKI group. On the other hand, cytokines are likely served as markers for preoperative severity of illness, an ongoing inflammatory process, or cardiac dysfunction. In the present research, preoperative levels of PCT, IL-2R, TNF-α were significantly different between AKI stage group and without AKI stage III group. These findings suggest that timing of surgery and alleviation of inflammation before surgery may assist with reducing postoperative AKI. AKI was most frequently diagnosed on day 2 after surgery because creatinine or BUN will delay to elevate after AKI occurs. But PCT, IL-2R, and TNF-α can reflect severe AKI as early as the first postoperative day, even before operation according our findings, which help us to decide offering appropriate treatment promptly.
Using SOFA and APACHE II scores for the severity of illness, a significant correlation was observed between these scores and serum levels of some analyzed biomarkers, such as PCT, TNF-α, and IL-2R. This was consistent with a positive correlation between inflammation cytokines levels and severity scores [Citation29]. Those biomarkers may predict severity of organ dysfunction earlier than SOFA score, and reflect the severity of organ dysfunction as well [Citation30]. Therefore, sequential measurement of biomarkers would help accurately identify the peak and may be an effective approach in clinical practice in patients after aortic dissection surgery.
This study contains a number of limitations. It is a single-center study and the number of patients studied is limited. Therefore, a prospective large-scale multicenter study is required to confirm our results. Second, our study has focused on inflammation to find predictors for postoperative adverse clinical outcomes in acute aortic dissection. However extensive inflammation activates the coagulation system and vice versa via crosstalk, and it would have been interesting if we add those markers for coagulation and platelet activation, as well as a potential relationship between these biomarkers will be further investigated in our future study.
Conclusion
In summary, aortic dissection surgery elicits a broad inflammatory response. Elevation of inflammatory mediators on early postoperative days provided important prognostic information for patients with acute aortic dissection. PCT, IL-2R may serve as valuable biomarkers in predicting mortality and AKI stage III, and IL-6 in predicting mortality in the population. Combination of these markers and APACHE II score would improve this capacity.
Author contributions
Hua Liu, Zhe Luo performed the research design, paper writing and revision; Lan Liu, Xiaomei Yang, Yamin Zhang, Ying Zhang performed the data collection; Guowei Tu, Guoguang Ma, Jili Zheng performed the data analysis; Duming Zhu, Chunsheng Wang contributed to the conception and design of the work, director of the work.
Acknowledgments
The authors thank all patients for participation in the study and the professionals of the ICU and Cardiac Surgery Department of Zhongshan Hospital for their assistance and support.
Disclosure statement
The authors declare that they there are no conflicts of interest regarding the publication of this paper.
Additional information
Funding
References
- Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350–358.
- Geirsson A, Ahlsson A, Franco-Cereceda A, et al. Hospital volumes and later year of operation correlates with better outcomes in acute type A aortic dissection. Eur J Cardiothorac Surg. 2018;53(1):276–281.
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type A dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63(17):1796–1803.
- Lawton JS, Moon MR, Liu J, et al. The profound impact of combined severe acidosis and malperfusion on operative mortality in the surgical treatment of type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155(3):897–904.
- McClure RS, Ouzounian M, Boodhwani M, et al. Cause of death following surgery for acute type A dissection: evidence from the Canadian Thoracic Aortic Collaborative. Aorta (Stamford). 2017;5(2):33–41.
- Pansini S, Gagliardotto PV, Pompei E, et al. Early and late risk factors in surgical treatment of acute type A aortic dissection. Ann Thorac Surg. 1998;66(3):779–784.
- Ko T, Higashitani M, Sato A, et al. Impact of acute kidney injury on early to long-term outcomes in patients who underwent surgery for type A acute aortic dissection. Am J Cardiol. 2015;116(3):463–468.
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973.
- Zhou H, Wang G, Yang L, et al. Acute kidney injury after total arch replacement combined with frozen elephant trunk implantation: incidence, risk factors, and outcome. J Cardiothorac Vasc Anesth. 2018;32(5):2210–2217.
- Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev. 2009;8(1):31–35.
- Yang Y, Xie J, Guo F, et al. Combination of C-reactive protein, procalcitonin and sepsis-related organ failure score for the diagnosis of sepsis in critical patients. Ann Intensive Care. 2016;6(1):51.
- Soni SS, Ronco C, Katz N, et al. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif. 2009;28(3):165–174.
- Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184.
- Dl T, Sigala F, Karaolanis G, et al. Cytokines as biomarkers of inflammatory response after open versus endovascular repair of abdominal aortic aneurysms: a systematic review. Acta Pharmacol Sin. 2018;39(7):1164–1175.
- Brocca A, Virzi GM, de Cal M, et al. Elevated levels of procalcitonin and interleukin-6 are linked with postoperative complications in cardiac surgery. Scand J Surg. 2017;106(4):318–324.
- Jiwaji Z, Nunn KP, Conway-Morris A, et al. Leukoreduced blood transfusion does not increase circulating soluble markers of inflammation: a randomized controlled trial. Transfusion. 2014;54(10):2404–2411.
- Hall R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(5):983–1033.
- Swartbol P, Truedsson L, Norgren L. The inflammatory response and its consequence for the clinical outcome following aortic aneurysm repair. Eur J Endovasc Surg. 2001;21(5):393–400.
- Kim T, Arnaoutakis GJ, Bihorac A, et al. Early blood biomarkers predict organ injury and resource utilization following complex cardiac surgery. J Surg Res. 2011;168(2):168–172.
- Bayer AL, Pugliese A, Malek TR. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol Res. 2013;57(1-3):197–209.
- Peveri P, Walz A, Dewald B, et al. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167(5):1547–1559.
- Choi JJ, McCarthy MW. Novel applications for serum procalcitonin testing in clinical practice. Expert Rev Mol Diagn. 2018;18(1):27–34.
- Liu H, Luo Z, Liu L, et al. Early kinetics of procalcitonin in predicting surgical outcomes in type A aortic dissection patients. Chin Med J (Engl). 2017;130(10):1175–1181.
- Pacini D, Pantaleo A, Di Marco L, et al. Risk factors for acute kidney injury after surgery of the thoracic aorta using antegrade selective cerebral perfusion and moderate hypothermia. J Thorac Cardiovasc Surg. 2015;150(1):127–133.
- Greenberq JH, Whitlock R, Zhang WR, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30(9):1519–1527.
- Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19(6):1106–1115.
- Jeeha R, Skinner DL, De Vasconcellos K, et al. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology (Carlton). 2018;23(12):1090–1095.
- Pecoits-Filho R, Heimbürger O, Bárány P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41(6):1212–1218.
- Bro-Jeppesen J, Kjaergaard J, Stammet P, et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1–8.
- Shimazui T, Matsumura Y, Nakada TA, et al. Serum levels of interleukin-6 may predict organ dysfunction earlier than SOFA score. Acute Med Surg. 2017;4(3):255–261.