Abstract
Objectives. Pericarditis, takotsubo cardiomyopathy and early repolarization syndrome (ERS) are well-known to mimic ST elevation myocardial infarction (STEMI). We aimed to study whether ECG findings of reciprocal ST depression, PR depression, ST-segment convexity or terminal QRS distortion can discriminate between ST elevation due to ischemia and non-ischemic conditions. Design. Eighty-five patients with STEMI and 94 patients with non-ischemic ST elevation were included. All patients had acute chest pain and at least 0.1 mV ST elevation. Presence of PR depression, ST-segment convexity, terminal QRS distortion or reciprocal ST depression was assessed in each ECG. Results. In anterior ST elevation, ST depression in lead II (≥0.025 mV) occurred in 40% of patients with STEMI but in none of the non-ischemic cases. In inferior ST elevation, ST depression in lead I (≥0.025 mV) was present in 83% of patients with STEMI but in none of the non-ischemic cases. Chest-lead PR depression was uncommon in STEMI (12%) compared to non-ischemic cases (38%; p < .001). Convex ST elevation occurred in 22% of STEMI cases and in 9% of non-ischemic cases (p = .01). Terminal QRS distortion was more prevalent in STEMI (40%) than in non-ischemic ST elevation (7%). In multivariable analysis, reciprocal ST depression was associated with an ischemic diagnosis, whereas ST depression in aVR and chest-lead PR depression were associated with a non-ischemic diagnosis. Conclusions. Identification of true STEMI among patients with different ST-elevation etiology may be improved by considering reciprocal ST depression, ST depression in aVR and chest-lead PR depression.
Introduction
In patients with acute chest pain, it is important to rapidly identify ST elevation myocardial infarction (STEMI) cases in order to restore the coronary circulation [Citation1,Citation2]. In general, this is done by determining whether the ECG fulfills STEMI criteria [Citation1–3]. However, these criteria have limited diagnostic accuracy, with low sensitivity for acute coronary occlusion [Citation4,Citation5]. “STEMI mimics” such as pericarditis, takotsubo cardiomyopathy, and early repolarization syndrome (ERS) are common diagnoses in cases of erroneous catheterization laboratory activation [Citation6–9].
Previous studies have reported different strategies to differentiate STEMI from single specific non-ischemic conditions [Citation8,Citation10–14]. However, in clinical reality, the differential diagnosis is not often restricted to two diagnoses.
We aimed to study whether reciprocal ST-segment changes, PR depression, ST-segment convexity or electrocardiographic findings of terminal QRS distortion can discriminate STEMI from non-ischemic conditions in a group of patients with different ST-elevation etiology.
Methods
This is a retrospective study in which patients were included from previously published studies [Citation15–17]. In this study, patients with chest pain, ST elevation ≥0.1 mV in at least one lead and QRS width <120 ms were included.
Ninety-five STEMI patients referred to acute primary percutaneous coronary intervention (PCI) were recruited from a study on pre-hospital oxygen treatment in STEMI patients [Citation16], of whom 85 patients met inclusion criteria (above). ECGs were recorded within 3 h from PCI (98% within 2 h). In case several ECGs were obtained, the ECG closest in time to PCI was included. Cardiovascular magnetic resonance imaging (CMR) was performed 2–6 d after the primary PCI on a Philips 1.5T Achieva or a Siemens 1.5T Avanto. T2-weighted images (triple inversion recovery imaging or T2-prepared steady-state free precession) were acquired in short-axis view, from base to apex of the left ventricle, to depict the myocardium at risk (MaR) [Citation18]. Analysis of MaR was performed using the freely available post-processing software Segment version 1.9 R3084 (http://segment.heiberg.se) [Citation19].
We recruited 95 patients with non-ischemic ST elevation and final diagnoses of perimyocarditis (n = 38), takotsubo cardiomyopathy (n = 22) or ERS (n = 35), of whom 94 patients met the inclusion criteria. The patients with perimyocarditis or takotsubo cardiomyopathy were recruited from a study where patients with these diagnoses had undergone diagnostic CMR. All patients with takotsubo cardiomyopathy also underwent acute coronary angiography without significant coronary stenoses and had imaging evidence of transient ventricular dysfunction with recovery at follow-up CMR or echocardiography [Citation15].
The 35 patients with ERS were included from the Evaluation of Unknown Predictors of Electrocardiographic Changes – a Transnational study (EXPECT) database [Citation17]. These patients were identified by evaluating all ECGs for ERS criteria in the database between October and December 2014 with at least 0.1 mV of ST elevation in any lead (except aVR), a negative troponin test, and no cardiac diagnosis at discharge (myocardial infarction, unstable angina, perimyocarditis). The ERS criteria used were QRS duration <120 ms and an end-QRS slur or notch on the downslope of a prominent R wave, at least 0.1 mV from baseline to nadir of the notch or slur in two contiguous leads [Citation20].
The ST-J amplitude was defined as the amplitude at the J point, relative to the PR junction in all 12 leads. Pathological Q waves were defined as a) any Q wave in leads V2–V3 ≥ 0.02 s, or b) QS complex in leads V2–V3, c) Q waves ≥0.03 s and 0.1 mV deep, or QS complex in any two anatomically contiguous leads of I, II, III, aVL, aVF or V4–V6, or d) an R wave ≥0.04 s in V1–V2 and R/S ≥ 1 with a concordant positive T wave in absence of a conduction defect [Citation3]. The presence of an S wave was defined as any deflection, following an R wave, below the PR junction.
J waves were defined as either QRS slurring or notching. QRS slurring was defined as a slowed inscription of the end of the QRS of a prominent R wave, initiated at least 0.1 mV above the baseline. QRS notching was defined as a positive deflection (entirely above the baseline) on the end of the downslope of a prominent R wave, at least 0.1 mV to nadir from the baseline [Citation20].
PR-segment depression was defined as depression of ≥0.05 mV compared to the TP segment, measured adjacent to QRS onset [Citation12,Citation13].
For analysis of terminal QRS distortion, all QRS complexes were designated to either a qR morphology (including qRs and qRS), Rs morphology (R-wave amplitude > S-wave amplitude, including Rs, Rsr, RsR and R), or other (QS and rS). All ECGs without pathological Q waves were then analyzed for fulfillment of terminal QRS distortion criteria. Terminal QRS distortion was considered present in leads with an initial R wave if the S wave and J wave were absent [Citation11], and in leads with qR configuration if the J-point elevation exceeded 50% of the R-wave amplitude [Citation21–23]. In this analysis, the inverted version of aVR (−aVR) was used instead of aVR.
Reciprocal ST depression was studied at two different cut-offs (0.025 and 0.05 mV). The cut-off of 0.025 mV has been used in previous studies on reciprocal ST-segment changes [Citation10]. A cut-off of 0.05 mV was also studied, in order to explore whether this cut-off would change diagnostic accuracy, for example by improving specificity. Patients with inferior ST elevation were analyzed regarding presence of reciprocal ST depression in aVL and I, V2 and V3. Patients with anterior ST elevation (V2–V4) were analyzed regarding presence of reciprocal ST depression in inferior leads (II, III and aVF). Further, patients with anterior ST elevation were analyzed regarding ST-segment changes in aVR, at the same two different cut-offs as above (0.025 and 0.05 mV), regarding both ST elevation and ST depression.
In this study, we included anonymized data from previous studies [Citation15–17] approved by the regional ethical review board. No additional personal data was registered for this study.
Besides ST-J amplitudes, which were retrieved from the previous studies, ECG interpretation regarding PR depression, ST-segment convexity, J waves and terminal QRS distortion was performed independently by three observers (TL, DM and IN) who were blinded to the clinical diagnosis. DM and IN were also blinded to the study design, and interpreted half of the ECGs each. In case of disagreement between TL and DM/IN, a decision was reached by consensus.
Continuous variables are presented as mean ± standard deviation or median and inter-quartile range as appropriate. The Shapiro–Wilk test was used to test for normality. Student’s t-test or Mann–Whitney U test was used for comparisons of means or medians between groups for normally or non-normally distributed variables, respectively. χ2 test was performed to compare proportions of prevalence of terminal QRS distortion, reciprocal ST-segment changes, PR depression and ST-segment convexity between groups. Odds ratios for the prediction of STEMI were calculated using a univariate binary logistic regression model. Variables with a p value <.05 at univariate analysis were entered into a multivariable model. Fleiss Kappa test was used to determine the level of inter-observer agreement. Sensitivity was calculated as true positives/number of patients with the condition tested for, specificity as true negatives/number of patients without the condition tested for, positive likelihood ratio (LR+) as sensitivity/(1 – specificity) and negative likelihood ratio (LR-) as 1 – sensitivity/specificity. Statistical analysis was performed using SPSS Statistics version 25 (SPSS Inc., IBM Corporation, Somers, NY). A p value of <.05 was considered statistically significant.
Results
Baseline characteristics of patients and general ECG variables are presented in . The prevalence of the various ECG findings in patients with STEMI and non-ischemic ST elevation are presented in and . Diagnostic accuracy (sensitivity, specificity and likelihood ratios) is summarized in and . A convex ST-segment was present in only 22% of STEMI patients but was still more common than in non-ischemic patients (9%; p =.01). PR depression occurred in 45% of non-ischemic cases (55% of pericarditis, 62% of Takotsubo cardiomyopathy and 23% of ERS patients) and in 31% of STEMI cases (p =.06). PR depression in the chest leads was more common in non-ischemic conditions (58%) than in STEMI (12%; p < .001). J waves occurred in 63% of non-ischemic conditions (47% of pericarditis, 29% of Takotsubo and 100% of ERS patients) compared to 27% of STEMI cases (p < .001).
Table 1. Baseline characteristics.
Table 2. Prevalence of ECG findings in patients with different ST-elevation etiology.
Table 3. Reciprocal ST-segment changes in patients with anterior or inferior ST elevation.
Table 4. ECG findings to be used to detect patients with STEMI: Sensitivity, specificity and likelihood ratio for an ischemic etiology.
Table 5. ECG findings to be used to detect non-ischemic patients: Sensitivity, specificity and likelihood ratio for a non-ischemic etiology.
In patients without pathological Q waves, both the S and J waves were absent in either V2 or V3 in only five patients with STEMI and two patients with non-ischemic ST elevation (p =.13). Terminal QRS distortion was more common in STEMI than non-ischemic conditions (40% vs. 7%, p < .001). There was no difference in MaR in STEMI patients positive (MaR 29% of the left ventricle (LV)) versus negative (32% of the LV) for terminal QRS distortion (p =.23).
Reciprocal ST depression was more common in patients with STEMI compared to patients with non-ischemic ST elevation (). In patients with anterior ST elevation, ST depression ≥0.025 mV in lead II occurred in 40% of STEMI patients, but in none of the non-ischemic cases (p < .001). In patients with inferior ST elevation, ST depression ≥0.025 mV in lead I occurred in 83% of STEMI patients, but in none of the non-ischemic cases (p < .001). For the majority of the leads studied, when a cut-off of 0.05 mV was used, reciprocal ST depression was less frequent in STEMI patients than when 0.025 mV was used (), and this resulted in a decreased sensitivity with only minor differences in specificity. For example, reciprocal ST depression in lead II (anterior ST elevation) occurred in 21% of STEMI patients when a cut-off of 0.05 mV used, but in 40% when 0.025 mV was used. Reciprocal ST depression in lead II was absent in all non-ischemic patients using either 0.025 or 0.05 mV as cut-off ().
In patients with anterior ST elevation, ST depression ≥0.025 mV in aVR was present in 80% of non-ischemic and in 30% of STEMI patients (p < .001). ST elevation in aVR, on the other hand, was present in 18% of STEMI patients (regardless of the location of ST-elevation), but in none of the non-ischemic patients (p < .001).
Results of the univariate and multivariable analysis of predictors of STEMI (vs. non-ischemic ST-elevation etiology) are presented in . At multivariable analysis adjusting for age and sex, reciprocal ST depression was the strongest independent predictor of ischemic ST elevation etiology (OR 9.9 (3.5–28.1), whereas chest-lead PR depression (OR 0.2 (0.05–0.5)) and ST depression in aVR (OR 0.2 (0.06–0.5)) were associated with a non-ischemic etiology.
Table 6. Univariate and multivariable predictors of ischemic STE.
Interobserver agreement was highest for the evaluation of absent S and J waves in leads with Rs (or R) configuration, with a κ of 0.96 (0.821.0). For the combined assessment of terminal QRS distortion, κ was 0.75 (0.59–0.90). The κ for PR depression was 0.80 (0.66–0.95), for J waves 0.78 (0.63–0.94) and for J-wave type (notch or slur) 0.73 (0.48–0.97). Interobserver agreement was lowest for ST-segment convexity (κ 0.68 (0.54–0.83)).
Discussion
In this study, we analyzed ECG findings other than ST-elevation amplitudes for differentiating STEMI from non-ischemic ST-elevation etiology ( and ), even in a heterogenous group of non-ischemic etiology. Reciprocal ST depression was more common in patients with STEMI than non-ischemic ST elevation and independently predicted an ischemic etiology. PR depression occurred in both STEMI and non-ischemic ST elevation, but PR depression in the chest leads was uncommon in patients with STEMI. Terminal QRS distortion and convex ST elevation were more common in STEMI than non-ischemic ST elevation, but convex ST elevation occurred only in a minority of STEMI patients.
Figure 1. ECGs (25 mm/s) from four patients with different ST elevation etiologies. (A) Patient with STEMI. ECG shows PR depression ≥0.05 mV in the limb leads, but not in the chest leads, and slight ST elevation in aVR. (B–D) Non-ischemic patients with ST elevation (B: perimyocarditis; C: takotsubo cardiomyopathy; D: ERS). Both (B) and (C) show PR depression in both limb leads and chest leads, in (D) minor PR depression is present in the limb leads, and PR depression ≥0.05 mV in lateral chest leads. All non-ischemic patients show some degree of ST depression in aVR.
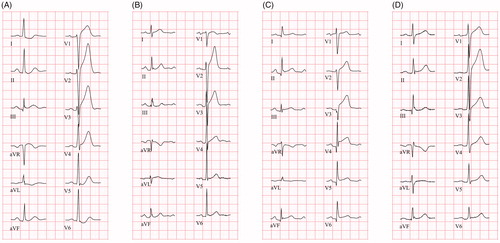
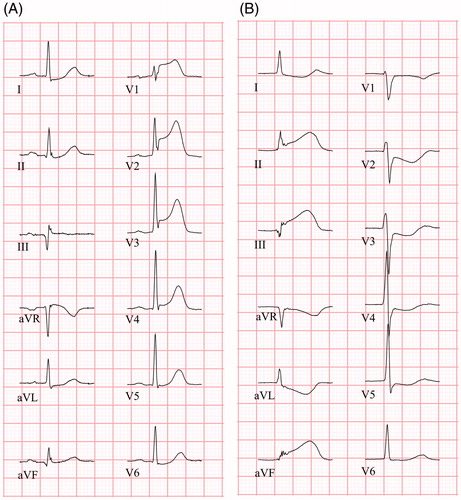
Figure 2. ECGs (25 mm/s) from two STEMI patients. (A) ECG shows concave ST elevation in V1 – V4 and ST depression in aVL, I and II. ST elevation is present in aVR. PR depression is absent in both limb leads and chest leads. Terminal QRS distortion is present in leads V2 and V3 (absent S and J wave in leads with ST elevation (Rs configuration)). (B) ECG shows concave ST elevation in inferior leads (II, aVF, III) with reciprocal ST depression in leads aVL and I, as well as precordial leads. PR depression is absent in both limb leads and chest leads. Terminal QRS distortion is present in aVF and III (ST elevation ≥50% of R-wave amplitude).
Correct ECG interpretation is essential for the management of patients with acute coronary syndrome, since treatment delay is associated with increased mortality [Citation24]. At the same time, it is important to avoid unnecessary coronary angiographies. False activation of the catherization laboratory is not uncommon and the non-ischemic diagnoses included in this study are common in these situations [Citation9,Citation25]. Of note, acute coronary angiography is often included in the evaluation of patients with takotsubo cardiomyopathy because of elevated cardiac biomarkers and its STEMI-like ECG appearance [Citation26,Citation27]. Besides ECG artifacts, perimyocarditis and ERS were the most common causes of false-positive software interpretations of STEMI in a prehospital study with >40.000 patients [Citation28].
In this study, ST depression in both aVL and I was more common in inferior STEMI than in inferior non-ischemic ST elevation. Similarly, ST depression in lead II was more common in anterior STEMI than in anterior non-ischemic ST elevation. Reciprocal ST depression was the strongest independent predictor of STEMI ().
In patients with suspected acute myocardial infarction, reciprocal changes in the ECG are important both for localizing the occlusion site and for assessment of infarct size and prognosis [Citation29–31], and also for differentiating STEMI from non-ischemic conditions. For example, it has been suggested that reciprocal ST depression in aVL in patients with inferior ST elevation can be used to discriminate between pericarditis and inferior STEMI [Citation10]; and to discriminate STEMI from takotsubo cardiomyopathy [Citation8].
Furthermore, in this study, ST depression in aVR was common in patients with non-ischemic ST elevation, but uncommon in patients with anterior STEMI. ST deviation in aVR has been suggested as an important discriminator between Takotsubo cardiomyopathy and anterior STEMI [Citation8,Citation32]. Although ST depression in aVR is more common in patients with Takotsubo cardiomyopathy than in STEMI patients, concerns have been raised that such ECG findings are not accurate enough to safely exclude STEMI [Citation33,Citation34].
In this study, PR depression in limb leads occurred in both non-ischemic ST elevation and STEMI but PR depression in chest leads was uncommon in STEMI (; ). In previous papers, PR depression has been described to occur in both pericarditis and Takotsubo cardiomyopathy [Citation12,Citation13]. In this study, PR depression was most common in Takotsubo patients (62%). Porela et al. [Citation13] compared electrocardiographic features in STEMI and acute perimyocarditis and also found chest-lead PR depression to be rare in STEMI patients (9%). In their study, PR depression in any lead in perimyocarditis was more prevalent (88%) than in our study (55%), even though the same electrocardiographic definition was used, perhaps due to (unknown) differences in disease duration. The PR depression is dynamic during the disease process and has been described to occur both earlier than ST elevation and have a shorter duration [Citation35]. Of note, PR depression in STEMI patients can be a sign of atrial infarction, most often seen in patients with occlusion of the right or the left circumflex coronary artery and is associated with an increased risk of supraventricular arrhythmias [Citation36]. Prominent PR depression (≥0.12 mV) in inferior leads in patients with acute inferior STEMI has been described to be associated with an increased risk of cardiac free-wall rupture and increased in-hospital mortality [Citation37].
Terminal QRS distortion was more prevalent in STEMI patients than in non-ischemic patients () in this study. ST changes during ischemia reflect altered repolarization due to changes in the action potentials in the ischemic myocardium [Citation38]. Depolarization changes are also present [Citation39], albeit often less evident and seldom used in in the routine diagnostic process. Terminal QRS distortion has been shown to predict poor prognosis [Citation21–23]. Absence of S waves in leads V1–V3, which normally have a terminal S wave, indicates severe ischemia [Citation21]. However, S waves can be absent in these leads also in ERS. Lee et al. [Citation11] showed that absence of both S and J wave in V2–V3 was highly specific for LAD occlusion when compared to patients with ERS. To determine the presence of an S wave requires very little effort and might thus be a clinically useful sign of ischemia. However, such findings in leads V2 or V3 were rare in our material (8% of STEMI patients, 2% of non-ischemic ST elevation). In this study, we combined “the Lee criterion” with the classical definition of terminal QRS distortion criteria in leads with qR configuration, but applied it to any lead with an initial R wave, not only V2–V3.
Although more prevalent in STEMI than in non-ischemic ST elevation (40 vs. 7%), terminal QRS distortion was not a statistically significant predictor at multivariable analysis (OR 2.7 (0.7–11.1)), ), perhaps due to the limited number of patients.
Although ST-segment convexity was more common in STEMI compared to non-ischemic conditions, it occurred in less than ¼ of STEMI patients. Previously, it has been suggested that STEMI is less likely in patients with concave ST elevation [Citation40]. This was dismissed by Smith et al., who reported that upwardly concave morphology was more common than convex morphology in patients with LAD occlusion [Citation41], which our study confirms.
This study confirms several previous observations on ECG findings that can be used to identify true STEMI. However, in contrast to previous studies which have compared findings in STEMI with those with ST elevation of a specific non-ischemic etiology, this study supports the use of selected criteria in situations with multiple non-ischemic differential diagnoses. In patients with ST elevation of unknown etiology, reciprocal ST depression increases the likelihood of an ischemic etiology, whereas presence of chest-lead PR depression and ST depression in aVR instead suggests a non-ischemic etiology. Although inferior ST depression seems to be specific for anterior STEMI, it lacks in sensitivity, and hence a STEMI diagnosis cannot be ruled out. In inferior ST elevation, on the other hand, ST depression in lead I, is highly sensitive and specific for STEMI, also expressed as a lower negative likelihood ratio than for reciprocal ST depression in anterior ST elevation. (0.2 vs. 0.6, ). Similarly, chest-lead PR depression seems to be specific for a non-ischemic diagnosis but lacks in sensitivity, whereas ST depression in aVR is highly sensitive for a non-ischemic diagnosis in anterior ST elevation but lacks in specificity. Thus, accurately differentiating STEMI from ST elevation of non-ischemic etiology requires a holistic ECG approach. Also, it should be taken into consideration when applying these ECG criteria that the consequences of delaying revascularization of a true STEMI may be far worse than performing an unnecessary coronary angiography.
A limitation to this study was that patients were included from different studies and not consecutively from the same setting. For example, STEMI patients were triaged for primary PCI whereas most of the non-ischemic patients were not. Nonetheless, all patients had acute chest pain and at least 0.1 mV ST elevation, which makes STEMI a relevant differential diagnosis in all these patients. Electrocardiographic changes during an ischemic process are dynamic. Comparisons of electrocardiographic changes, such as terminal QRS distortion, and CMR to assess myocardium at risk are therefore difficult. For example, in case of a spontaneous opening of a previously occluded artery, electrocardiographic changes will subside whereas MaR by CMR will remain the same.
Different ERS patterns exist, for example with lateral or inferior J waves and ST elevation [Citation42]. Even though ERS patients were randomly selected from an ED population, all ERS patients had ST elevation in V2–V4 and the typical lead for maximal ST elevation was V2 (66%). The results in this study regarding ERS may, therefore, not be applicable to patients with other ST elevation patterns.
Several other ECG criteria have been suggested to be included in the differential diagnosis of ST elevation, such as the QT interval and QRS amplitudes [Citation43], but these were not assessed in this paper.
Blinded interpretation of ECG parameters was made only regarding terminal QRS distortion, PR depression and ST-segment convexity, not regarding ST-J amplitudes. Also, although TL was blinded to the clinical diagnosis during interpretation, he was not unfamiliar with the ECGs from previous studies [Citation15], and he identified the ERS patients in the EXPECT database. However, the other two ECG interpreters were blinded to both study design and final diagnoses, and in most cases inter-rater agreement was strong, suggesting that the impact on the results was minor. Reciprocal ST depression was based on ST-J amplitudes from the previous studies, most of them from automated measurements.
In this study, a validation group for the ECG signs found to be useful in the differentiation of ischemic and non-ischemic ST-elevation was lacking, and the findings, therefore, need to be confirmed in larger studies.
Conclusion
Identification of true STEMI among patients with different ST-elevation etiologies may be improved by considering different ECG changes in addition to the ST elevation; primarily reciprocal ST depression, ST depression in aVR and PR depression in the chest leads.
Disclosure statement
UE and HE have received funding from Lund University (ALF grants), funding from Region Skåne, Sweden and the Swedish Heart-Lung foundation. The other authors report no conflict of interest.
Additional information
Funding
References
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177.
- O’Gara PT, Kushner FG, Ascheim DD, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425.
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2018;40:237–269.
- Martin TN, Groenning BA, Murray HM, et al. ST-segment deviation analysis of the admission 12-lead electrocardiogram as an aid to early diagnosis of acute myocardial infarction with a cardiac magnetic resonance imaging gold standard. J Am Coll Cardiol. 2007;50:1021–1028.
- Hillinger P, Strebel I, Abächerli R, et al. Prospective validation of current quantitative electrocardiographic criteria for ST-elevation myocardial infarction. Int J Cardiol. 2019;292:1–12.
- Huang HD, Birnbaum Y. ST elevation: differentiation between ST elevation myocardial infarction and nonischemic ST elevation. J Electrocardiol. 2011;44:494.e1–494.e12.
- Pollak P, Brady W. Electrocardiographic patterns mimicking ST segment elevation myocardial infarction. Cardiol Clin. 2012;30:601–615.
- Frangieh AH, Obeid S, Ghadri JR, et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J Am Heart Assoc. 2016;5:e003418.
- Larson DM, Menssen KM, Sharkey SW, et al. False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760.
- Bischof JE, Worrall C, Thompson P, et al. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016;34:149–154.
- Lee DH, Walsh B, Smith SW. Terminal QRS distortion is present in anterior myocardial infarction but absent in early repolarization. Am J Emerg Med. 2016;34:2182–2185.
- Zorzi A, Baritussio A, ElMaghawry M, et al. Differential diagnosis at admission between Takotsubo cardiomyopathy and acute apical-anterior myocardial infarction in postmenopausal women. Eur Heart J Acute Cardiovasc Care. 2016;5:298–307.
- Porela P, Kyto V, Nikus K, et al. PR depression is useful in the differential diagnosis of myopericarditis and ST elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17:141–145.
- Ogura R, Hiasa Y, Takahashi T, et al. Specific findings of the standard 12-lead ECG in patients with ‘Takotsubo’ cardiomyopathy: comparison with the findings of acute anterior myocardial infarction. Circ J. 2003;67:687–690.
- Lindow T, Pahlm O, Olson CW, et al. Diagnostic accuracy of the electrocardiographic decision support – myocardial ischaemia (EDS-MI) algorithm in detection of acute coronary occlusion. Eur Heart J Acute Cardiovasc Care. 2018.
- Khoshnood A, Carlsson M, Akbarzadeh M, et al. Effect of oxygen therapy on myocardial salvage in ST elevation myocardial infarction: the randomized SOCCER trial. Eur J Emerg Med. 2018;25:78–84.
- Schade Hansen C, Pottegard A, Ekelund U, et al. Association between QTc prolongation and mortality in patients with suspected poisoning in the emergency department: a transnational propensity score matched cohort study. BMJ Open. 2018;78:e020036.
- Carlsson M, Ubachs JFA, Hedström E, et al. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–576.
- Heiberg E, Sjögren J, Ugander M, et al. Design and validation of Segment–freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1.
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol. 2015;66:470–477.
- Birnbaum Y, Sclarovsky S. The grades of ischemia on the presenting electrocardiogram of patients with ST elevation acute myocardial infarction. J Electrocardiol. 2001;34:17–26.
- Ringborn M, Birnbaum Y, Nielsen SS, et al. Pre-hospital evaluation of electrocardiographic grade 3 ischemia predicts infarct progression and final infarct size in ST elevation myocardial infarction patients treated with primary percutaneous coronary intervention. J Electrocardiol. 2014;47:556–565.
- Garcia-Rubira JC, Perez-Leal I, Garcia-Martinez JT, et al. The initial electrocardiogram pattern is a strong predictor of outcome in acute myocardial infarction. Int J Cardiol. 1995;51:301–305.
- Solhpour A, Chang KW, Arain SA, et al. Ischemic time is a better predictor than door-to-balloon time for mortality and infarct size in ST-elevation myocardial infarction. Cathet Cardiovasc Intervent. 2016;87:1194–1200.
- McCabe JM, Armstrong EJ, Kulkarni A, et al. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention-capable centers: a report from the Activate-SF registry. Arch Intern Med. 2012;172:864–871.
- de Bliek EC. ST elevation: differential diagnosis and caveats. A comprehensive review to help distinguish ST elevation myocardial infarction from nonischemic etiologies of ST elevation. Turk J Emerg Med. 2018;18:1–10.
- Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;739:2047–2062.
- Bosson N, Sanko S, Stickney RE, et al. Causes of prehospital misinterpretations of ST elevation myocardial infarction. Prehosp Emerg Care. 2017;21:283–290.
- Willems JL, Willems RJ, Willems GM, et al. Significance of initial ST segment elevation and depression for the management of thrombolytic therapy in acute myocardial infarction. European Cooperative Study Group for Recombinant Tissue-Type Plasminogen Activator. Circulation. 1990;82:1147–1158.
- Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol. 1999;34:389–395.
- Wagner GS, Macfarlane P, Wellens H, et al; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:1003–1011.
- Kosuge M, Kimura K. Electrocardiographic findings of Takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J Electrocardiol. 2014;47:684–689.
- Vervaat FE, Christensen TE, Smeijers L, et al. Is it possible to differentiate between Takotsubo cardiomyopathy and acute anterior ST-elevation myocardial infarction? J Electrocardiol. 2015;48:512–519.
- Johnson NP, Chavez JF, Mosley WJ, et al. Performance of electrocardiographic criteria to differentiate Takotsubo cardiomyopathy from acute anterior ST elevation myocardial infarction. Int J Cardiol. 2013;164:345–348.
- Chan TC, Brady WJ, Pollack M. Electrocardiographic manifestations: acute myopericarditis. J Emerg Med. 1999;17:865–872.
- Lu MLR, De Venecia T, Patnaik S, et al. Atrial myocardial infarction: a tale of the forgotten chamber. Int J Cardiol. 2016;202:904–909.
- Jim MH, Siu CW, Chan AO, et al. Prognostic implications of PR-segment depression in inferior leads in acute inferior myocardial infarction. Clin Cardiol. 2006;29:363–368.
- Surawicz B. Ventricular repolarization in myocardial ischemia and myocardial infarction: theory and practice. In: Macfarlane PW, van Oosterom A, Pahlm O, et al., editors. Comprehensive electrocardiology. London: Springer; 2010. p. 803–831.
- Surawicz B. Reversible QRS changes during acute myocardial ischemia. J Electrocardiol. 1998;31:209–220.
- Antman EM, Canadian Cardiovascular Society, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:671–719.
- Smith SW. Upwardly concave ST segment morphology is common in acute left anterior descending coronary occlusion. J Emerg Med. 2006;31:69–77.
- Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558.
- Driver BE, Khalil A, Henry T, et al. A new 4-variable formula to differentiate normal variant ST segment elevation in V2-V4 (early repolarization) from subtle left anterior descending coronary occlusion – adding QRS amplitude of V2 improves the model. J Electrocardiol. 2017;50:561–569.
