Abstract
Objective. Aortic valve sclerosis (AVS) is closely related to endothelial dysfunction. The association of AVS with contrast-induced nephropathy (CIN) is unknown. We planned to investigate the relationship of AVS besides known parameters with CIN. Design. Baseline characteristics, biochemical values, and AVS of 292 consecutive patients with acute coronary syndrome (ACS) that underwent percutaneous coronary intervention (PCI) were analyzed. Results. Fifty-three patients (18.2%) had CIN. Patients with CIN were older, less likely to be smokers, and had more prevalent prior bypass surgery, higher Mehran score, creatinine, and uric acid concentrations than those without CIN. AVS was more prevalent in patients with CIN. Logistic regression analysis including all related parameters identified Mehran score (OR = 1.036, p = .033), uric acid concentration (OR = 1.244, p = .023), and AVS (OR: 2.223, p = .027) as independent predictors of CIN. Conclusion. AVS is independently associated with CIN in patients with acute coronary syndrome undergoing percutaneous coronary intervention. AVS may help to identify high-risk patients for CIN, who would benefit from preventive measures.
Introduction
Percutaneous coronary intervention (PCI), the most common revascularization option in patients with acute coronary syndrome (ACS), not only relieves symptoms but also improves prognosis [Citation1]. Since PCI necessitates the use of a contrast agent, contrast-induced nephropathy (CIN) is one of the complications that is not related to the coronary intervention primarily. CIN may have varying severity, from mild and temporary renal dysfunction to severe renal failure that requires dialysis. Additionally, CIN is related to longer hospital stay, increased cost, higher morbidity, and both in-hospital and long-term cardiovascular mortalities [Citation2]. Thus, it is imperative to identify patients that are at high risk for CIN and take preventive measures [Citation3].
Previous studies identified chronic renal failure as the main determinant of CIN besides several other risk factors. These factors include advanced age, contrast volume, the presence of diabetes mellitus (DM), hypovolemia, hypotension, congestive heart failure, anemia, diastolic dysfunction, the use of angiotensin-converting enzyme inhibitors and nephrotoxic drugs, hyperuricemia, hemoglobin A1c, and Mehran score [Citation4–7].
Echocardiography is widely used as a diagnostic tool in routine cardiology practice. So far, certain echocardiographic parameters such as inferior caval vein dimensions, left-ventricular systolic, and diastolic function have been related to CIN [Citation6,Citation8]. A previous study revealed that aortic valve sclerosis (AVS) is closely related to endothelial dysfunction [Citation9]. The association of AVS with CIN remains unknown. Since CIN is related to several risk factors associated with endothelial dysfunction, we hypothesized that CIN might be related to AVS as well. Therefore, we planned to investigate the relationship of AVS with CIN in patients with ACS that underwent PCI.
Methods
Patient population and study protocol
Our cohort study was prospective and observational. We included 292 consecutive patients (237 males, 55 females) who underwent PCI due to ACS in Recep Tayyip Erdoğan University Education and Training Hospital between February and September 2018. The study was performed in accordance with the principles stated in the Declaration of Helsinki. The local ethics committee approved the study protocol.
The diagnosis of ACS was defined by the current guidelines based on cardiac symptoms, electrocardiographic appearance, and biomarkers reflecting myocardial damage [Citation10]. Patients who accepted intervention and gave informed consent were included in the study. PCI was performed within 24 h in subjects with unstable angina pectoris and non-ST segment elevation myocardial infarction (NSTEMI), whereas patients with clinical instability and ST-segment elevation myocardial infarction (STEMI) underwent PCI as soon as possible. Medical treatment of patients was given in accord with the most recent guidelines [Citation10]. All patients with NSTEMI-ACS without signs and symptoms of congestive heart failure received intravenous hydration with isotonic saline at 0.5–1 mL/kg per hour for 12 h started prior to intervention according to left-ventricular ejection fraction.
A cardiologist assessed all subjects before PCI, and noted vital signs, medical history, basal characteristics including the presence of HT, DM, previous revascularization, previous cerebrovascular event, peripheral artery disease, family history of premature coronary artery disease, and smoking. Patients underwent echocardiography within the first two days of hospitalization.
Hypertension (HT) was defined as the active use of antihypertensive drugs or documentation of blood pressure more than 140/90 mmHg. Diabetes mellitus was acknowledged if fasting plasma glucose levels over 126 mg/dl or glucose level over 200 mg/dl at any measurement or active use of antidiabetic treatment. Patients who were using tobacco products on admission and those who quitted smoking within the last year were considered as smokers. Mehran score was assessed with the help of an online calculator (http://www.pmidcalc.org/?sid=15464318&newtest=Y) [Citation7]. Body mass index (BMI) was determined by the following formula: BMI = weight (kg)/height2 (m).
Primary endpoint
CIN was defined as an increase in the serum creatinine concentration by 0.5 mg/dL or 25% elevation from baseline in the first 48 to 72 h after PCI. Renal insufficiency was defined as a glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, estimated using the modification of diet in renal disease (MDRD) formula [Citation11]. Patients were stratified into two groups according to CIN. We checked creatinine values daily during hospitalization, and the highest value was acknowledged as peak creatinine.
Exclusion criteria
The exclusion criteria were renal failure with eGFR of <30 mL/min/1.73 m2, malignancy, sepsis, pulmonary embolism, use of another method for revascularization, and patients with possible infections associated with acute kidney injury. We also excluded patients who died in 48 h, patients who required contrast agent for different reasons, patients who did not have echocardiographic examination, and serum creatinine in the first 72 h, and patients with cardiogenic shock.
Selective coronary angiography and PCI procedure
Selective coronary angiography and the duration of PCI were in accordance with the current guidelines and were performed using the Judkins technique. Multiple views were obtained in all patients, with visualization of the left anterior descending and left circumflex coronary artery in at least four views, and the right coronary artery in at least two views. All procedures were performed through the femoral or radial approach by experienced interventional cardiologists. Nonionic, low-osmolar contrast medium (iohexol, Omnipaque 350 mg/mL; GE Healthcare, Cork, Ireland) was used. The amount of contrast agent was noted. The severity and length of the culprit lesion were calculated using angiographic software using the best view. Ad hoc PCI was performed provided that the patient did not have an indication for coronary artery bypass graft (CABG) surgery. The heart team discussed all patients with multi-vessel disease.
Laboratory assays
Cardiac biomarker levels including, troponin-I, admission glucose, fibrinogen, and inflammatory markers including leukocytes, were measured at our emergency department and used in the analyses as admission values. The lipid samples were drawn by venipuncture to perform routine blood chemistry after fasting for at least 8 h. Glucose, creatinine, and lipid profile were determined by standard methods. White blood cell (WBC, leukocyte) counts were obtained from an automated cell counter (Coulter Gen-S, COULTER Corp, Miami, USA). CK-MB, troponin, and troponin concentrations were measured every 12 h in the first 72-hour period to demonstrate the peak values. The peak value was used in the study. Because troponin levels above 50 ng/mL are stated by our laboratory as >50 ng/mL, they were entered as 50 ng/mL for statistical analysis.
Echocardiography
Patients were imaged in the left lateral decubitus position with commercially available systems using a Philips IE33 system (Philips, Andover, MA, USA) with a 2.5–3.5-MHz transducer. Two experienced cardiologists performed echocardiography according to the current recommendations [Citation12]. Ejection fraction was measured using the modified Simpson method.
The morphology and function of the aortic valve, as well as the presence of aortic valve sclerosis, was assessed. AVS was identified as thickening or calcification of the aortic valve leaflets without a significant trans-valvular gradient (maximum aortic velocity ≤2.5 m/s), in the absence of rheumatic heart disease [Citation13].
Statistical analysis
The Statistical Program for Social Sciences (SPSS for Windows 15, Inc., Chicago, IL, USA) was used for all statistical calculations. Nominal variables were presented as the number of cases with percentages and continuous variables as mean ± SD. Data were tested for normal distribution using the Shapiro–Wilk test. The Chi-square or Fisher’s exact tests were used for comparison of categorical variables between two groups. Continuous variables were compared using Student’s t-test and the Mann–Whitney U-test where appropriate. Univariate correlation analysis was performed by Pearson’s test to identify variables that potentially affect CIN. Variables with a p value of <.05 in the univariate correlation analysis were included in a multivariate stepwise regression analysis model to assess the independent determinants of CIN. Since gender and eGFR are already included in the Mehran score, they were not entered into the multivariate analysis. All tests of significance were two-tailed. Statistical significance was defined as p < .05
Results
Baseline characteristics
Among 292 patients with ACS that underwent PCI, 53 (18.2%) had CIN. Patients with CIN were older (67.4 ± 13.1 vs. 61.8 ± 10.9, p = .001), less likely to be smokers (28.3% vs. 46.4%, p = .011), and had more prevalent prior bypass surgery (13.2% vs. 4.6%, p = .027). Mehran score was higher in patients with CIN compared to those without (p = .016) (). Contrast volume did not differ between groups. Previous use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was also similar.
Table 1. Characteristics of the study population.
Laboratory values
Patients with CIN had higher admission creatinine (1.04 ± 0.27 vs. 0.97 ± 0.32 mg/dl, p = .037) and uric acid (6.4 ± 1.7 vs. 5.8 ± 1.4 mg/dl, p = .008) concentrations compared to subjects without CIN. As expected those with CIN had lower estimated GFR (eGFR) than patients without CIN (73 ± 19 vs. 81.6 ± 19 mL/min/1.73 m2, p = .006). Other values were not different between groups.
Echocardiographic parameters
Left ventricular systolic and diastolic functions were similar between groups. AVS (r = 0.285 p = .002) was more prevalent among patients with CIN (75.5% vs. 51.7%, p = .001).
Multivariate analysis
Logistic regression analysis including all related factors identified Mehran score (OR = 1.036, 95% CI: 1.010–1.062, p = .033), uric acid concentration (OR = 1.244, 95% CI: 1.032–1.526, p = .023), and AVS (OR: 2.223, 95% CI: 1.095–4.515, p = .027) as independent predictors of CIN ().
Table 2. Logistical regression analysis for prediction of CIN.
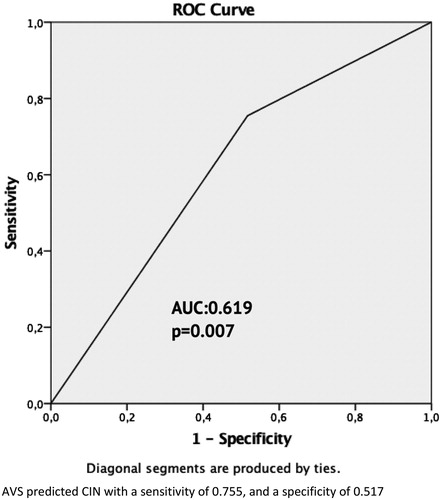
We performed receiver under curve (ROC) analysis, and demonstrated that AVS predicted CIN with a sensitivity of 0.755 and a specificity of 0.517(AUC = 0.619, p = .007) ().
Discussion
We discovered that besides previously known parameters including the Mehran score and uric acid, echocardiographically proven AVS is independently associated with CIN in patients with ACS, who underwent PCI. As far as we know, our study is the first to demonstrate an independent relation of AVS with CIN.
AVS is defined as focal or diffuse thickening and/or calcification of aortic valve without significant obstruction. Although increased aortic velocity is commonly observed, maximum velocity should be below 2.5 m/s by definition. The prevalence is reported as 29% in subjects over 65 years, which increases in advanced ages [Citation13]. Besides aging, AVS is more common in patients with higher cardiovascular risk. Chandra and coworkers demonstrated AVS in 49% of patients who presented with acute chest pain to the emergency unit [Citation14]. Our patients with CIN had 75.5% AVS and those without had 51.7%. Given the fact that we included a high cardiovascular risk group, this prevalence is reasonable and in line with the literature. Despite its wide prevalence, AVS is not a benign condition. Data suggest that AVS may progress to aortic stenosis and have been associated with increased myocardial infarction, heart failure, all-cause mortality, and cardiovascular mortality [Citation15,Citation16].
There is not any information in the current literature regarding the relationship between CIN and AVS. Former studies identified the association of AVS with male gender, smoking, hypertension, hypercholesterolemia, and diabetes, all of which are risk factors of coronary artery disease [Citation17]. Accordingly, histopathological studies documented an active inflammatory process and prominent calcification with similarities to atherosclerosis including lipid accumulation [Citation18]. Moreover, Yang et al. revealed that the presence and severity of AVS is associated with alteration of calcium and phosphate metabolism in patients with normal or mildly impaired renal function [Citation19]. We previously showed that AVS is closely related to flow-mediated dilation, a marker of endothelial dysfunction [Citation9]. Therefore, AVS seems to be an active process that includes atherosclerotic risk factors, deranged mineral metabolism, and inflammation. Similarly, CIN has also been related to renal dysfunction and inflammation [Citation20]. If we speculate, since CIN and AVS share common factors and similar atherosclerotic mechanisms, AVS may not be a cause CIN, but a possible marker of shared factors and pathogenesis. AVS is easily identifiable by echocardiography even at bedside. Therefore, we think that the presence of AVS in a patient with ACS, who is scheduled for PCI, may alert clinicians for preventive action like intravenous hydration.
We could not document an association between CIN and contrast volume in our study. Han et al. revealed that diastolic dysfunction, HbA1c, and Mehran score were independently related to CIN in 379 patients with acute MI. CIN was observed in 23% of patients in their study [Citation6]. The results of this study support our findings. Han and coworkers also could not document an association between contrast volume and CIN. We may speculate that renal susceptibility rather than contrast volume is important in CIN. A recent study including 2000 patients with STEMI stated that the risk of CIN is related mainly with age, baseline estimated GFR, heart failure, and hemodynamic instability but not with contrast volume [Citation4].
Our study has several limitations, the most important being sample size. Patients with STEMI underwent urgent revascularization and did not have prophylactic hydration like the rest of the group. We also excluded patients with cardiogenic shock and patients with eGFR < 30 mL/min/m2, which preclude generalization of our results. Moreover, our study is cross-sectional and does not implicate causality. However, all patients with an ACS require echocardiographic imaging. Our findings may help to identify patients at high risk for CIN, who would benefit from preventive measures.
Conclusion
Aortic sclerosis is an independent predictor of contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. As AVS may be assessed simply and easily with bedside echocardiography, the presence of AVS may help to identify high-risk patients for CIN, who would benefit from preventive measures
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Neumann FJ, Sousa-Uva M, Ahlsson A. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87–165.
- Senoo T, Motohiro M, Kamihata H, et al. Contrast-induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105(5):624–628.
- Perrin T, Descombes E, Cook S. Contrast-induced nephropathy in invasive cardiology. Swiss Med Wkly. 2012;142:w13608.
- Caspi O, Habib M, Cohen Y, et al. Acute kidney injury after primary angioplasty: is contrast-induced nephropathy the culprit? J Am Heart Assoc. 2017 Jun 24;6(6):e005715.
- Barbieri L, Verdoia M, Schaffer A, et al. Pre-diabetes and the risk of contrast induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. Diabetes Res Clin Pract. 2014;106(3):458–464.
- Han B, Li Y, Dong Z, et al. Diastolic dysfunction predicts the risk of contrast-induced nephropathy and outcome post-emergency percutaneous coronary intervention in AMI patients with preserved ejection fraction. Heart Vessels. 2018;33(10):1149–1158.
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004 6;44(7):1393–1399.
- Gungoren F, Besli F, Tanriverdi Z, et al. Inferior vena cava assessment can predict contrast-induced nephropathy in patients undergoing cardiac catheterization: a single-center prospective study. Echocardiography. 2018;35(12):1915–1921.
- Erdogan T, Cetin M, Kocaman SA, et al. Aortic valve sclerosis is a high predictive marker of systemic endothelial dysfunction in hypertensive patients. Herz. 2013;38(8):915–921.
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270.
- Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341(3):142–147.
- Chandra HR, Goldstein JA, Choudhary N, et al. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 2004;43(2):169–175.
- Cosmi JE, Kort S, Tunick PA, et al. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162(20):2345–2347.
- Shah SJ, Ristow B, Ali S, et al. Acute myocardial infarction in patients with versus without aortic valve sclerosis and effect of statin therapy (from the Heart and Soul Study). Am J Cardiol. 2007;99(8):1128–1133.
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634.
- Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853.
- Yang ZK, Ying C, Zhao HY, et al. Mineral metabolism disturbances are associated with the presence and severity of calcific aortic valve disease. J Zhejiang Univ Sci B. 2015;16(5):362–369.
- Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast-induced nephropathy. BJR. 2014 Sep;87(1041):20130738.