Abstract
Objectives. There is limited knowledge of atrial fibrillation (AF) incidence among the very old. Data from longitudinal cohort studies may give us a better insight. The aim of the study was to investigate the incidence rate and prevalence of AF, as well as the impact of AF on mortality, in the general population, from 70 to 100 years of age. Design. This was a population-based prospective cohort study where three representative samples of 70-year-old men and women (n = 2,629) from the Gerontological and Geriatric Populations Studies in Gothenburg (H-70) were included between 1971 and 1982. The participants were examined at age 70 years and were re-examined repeatedly until 100 years of age. AF was diagnosed according to a 12-lead electrocardiogram (ECG) recording at baseline and follow-up examinations, from the Swedish National Patient Register (NPR), or from the Cause of Death Register. Results. The cumulative incidence of AF from 70 to 100 years of age was 65.6% for men and 52.8% for women. Mortality was significantly higher in participants with AF compared with those without, rate ratio (RR) 1.92 (95% CI 1.73–2.14). In a subgroup analysis comprising only participants with AF diagnosed by ECG at screening, the RR for death was 1.29 (95% C.I: 1.03–1.63). Conclusions. Among persons surviving to age 70, the cumulative incidence of AF was over 50% during follow-up. Mortality rate was twice as high in participants with AF compared to participants without AF. Among participants with AF first recorded at a screening examination, the increased risk was only 29%.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia and every fourth person is expected to suffer from the disease during his/her lifetime [Citation1–4]. It is associated with increased morbidity and mortality [Citation5,Citation6] and the risk of stroke is five times higher among subjects with AF [Citation7]. About 30% of ischaemic strokes are considered to be caused by a cardiac embolism [Citation8] and the majority of these are due to AF [Citation9]. Both permanent and paroxysmal AF increase the risk of stroke to the same extent [Citation1,Citation10].
The prevalence of AF is low before the age of 50 years and it then increases with increasing age, to almost 20% at ages above 80 years [Citation1–3]. It is higher in men than in women, but the difference appears to decrease with age [Citation11]. Studies published in recent years have shown an increasing prevalence over time, the reason for this could be improved diagnostic procedures, but increased survival in ischemic heart disease and heart failure might also contribute [Citation12,Citation13].
Common diseases in older individuals, such as hypertension, valvular heart disease and diabetes, are often associated with AF [Citation14–16] and the excess mortality rate among patients with AF is mainly seen among those with other concomitant diseases, such as stroke, heart failure, ischaemic heart disease and chronic obstructive pulmonary disease [Citation6,Citation17–19].
Most previous epidemiological studies of AF have focused on populations up to the age of 80 years. Knowledge of the incidence of AF in the highest age groups is still limited. The extent to which AF increases the risk of mortality among the oldest people in the population is also unclear [Citation20]. During the last few decades, survival in the highest age groups has increased significantly and the proportion of very old people in the population is rising. Studies of age-related morbidity and mortality are of major importance in order to provide older patients with better care and thus optimise their health and well-being [Citation21].
The aims of this study were (1) to investigate the incidence rate and the prevalence of AF between the age of 70 and 100 years in men and women and (2) to investigate the mortality rates in men and women with AF, compared with men and women without AF in this age range.
Methods
The Gerontological and Geriatric Population Studies in Gothenburg, Sweden (H70), include six cohorts of men and women followed longitudinally from the age of 70 years. This report is restricted to the first three cohorts included between 1971 and 1982 and followed over a period of 30 years between the ages of 70 and 100 years. All the participants were living in the City of Gothenburg, Sweden, with approximately 500,000 inhabitants at the time of the study. The first examination started in 1971 with a systematic sampling of 30% of the 70-year-old population in Gothenburg [Citation22], referred to here as Cohort 1. The sample comprised 1,148 subjects. The response rate was 85% and 449 men and 525 women participated. This sample was re-examined at 75, 79, 81, 82, 83, 85, 88, 90, 92, 95, 97, 99 and 100 years of age.
In 1976-77, a second population sample of 70-year-olds (n = 1,281) was invited to participate. Eighty-one percent participated (474 men and 562 women) and were examined [Citation23]. The survivors were re-examined at age 75 and 79. This sample is referred to as Cohort 2.
In 1982, a third population sample of 70 year olds (n = 806) was invited and 619 subjects, 302 men and 317 women (participation rate 77%), were examined as part of the Intervention Study of Elderly in Gothenburg [Citation24]. The survivors were re-examined at age 73. At age 76, the survivors were invited to a re-examination, together with the survivors from a control group identified at age 70. A total of 293 men and 356 women were investigated at age 76 and re-examined at 86 years. This sample is referred to as Cohort 3.
All three cohorts have been shown to be representative of the general population in Gothenburg. Further details of the sampling procedure and participation rates at all examinations have previously been presented elsewhere [Citation22–24].
Each investigation comprised a broad multidisciplinary investigation including an interview with the registration of social factors and lifestyle-related variables, medical history, registration of medication and a clinical examination including a physical examination by a physician, blood pressure measurements, an electrocardiogram (ECG) and laboratory examinations. The interview included questionnaires with information about earlier and current smoking habits. Socioeconomic data and drug consumption were registered during a home call by a registered nurse. All the other examinations were performed during a visit to the out-patient department at the hospital. Subjects who smoked or had stopped smoking during the previous year were defined as current smokers. Ex-smokers who had stopped smoking more than a year before the examination and subjects who had never smoked were defined as non-smokers.
A 12-lead ECG was recorded in the supine position at a paper speed of 50 mm/s, and coded according to the Minnesota Code. Interobserver variation tests were performed between the investigators responsible for the coding and no significant differences were found. Since 1981, three investigators have been responsible for all the coding. AF was defined as Minnesota Code 8.3.
Blood pressure was measured in the right arm in a seated position after five minutes’ rest, to the nearest 2 mmHg. Diastolic blood pressure was defined as Korotkoff phase 5. A mercury manometer was used for the registrations. Hypertension was defined as pharmacological treatment for hypertension and/or systolic blood pressure of ≥160 mmHg and/or diastolic blood pressure of ≥95 mmHg at the time of examination. Body mass index (BMI) was calculated as weight/height [Citation2] (kg/m2).
Diabetes mellitus was diagnosed as previously diagnosed by physicians, being on any anti-diabetic treatment or having two fasting venous or capillary whole blood glucose values of ≥7.0 mmol/L. Serum cholesterol was measured using standard methods at the laboratory at Sahlgrenska University Hospital.
Since 1978, data from the Swedish National Patient Register (NPR) have been available and contain all discharge diagnoses from Swedish hospitals, with full coverage from Gothenburg hospitals from 1980 and nationwide coverage from 1987. The discharge diagnoses are classified according to The International Classification of Diseases, 9th revision (ICD-9) and, from 1997, 10th revision (ICD-10). AF was defined as ICD-9: 427.3 or ICD-10: I48. A diagnosis of AF from the NPR has been validated as reliable with a positive predictive value (PPV) of 97% [Citation25].
Mortality data were collected from the Swedish National Board of Health and Welfare, register of causes of death.
A participant was considered to have AF during follow-up if: (a) an AF was diagnosed by ECG at screening examination, (b) AF was diagnosed by the NPR or (c) AF was diagnosed by the National Board of Health and Welfare, register of causes of death. Follow-up was until death or 100 years of age for all participants.
Statistical analysis
Parameters of survival analysis were estimated using Poisson regression models. The results from this model, presented as rate ratios (RRs) for mortality, are comparable to the corresponding results from Cox proportional hazards model, but, for the current analysis, the use of Poisson regression was considered to offer some practical advantages.
To perform the calculations of a Poisson survival model, the risk time for each individual is divided into small time intervals where the outcome event is observed to have either happened or not. All intervals except the last for each individual are event free. The last interval may or may not have the event, depending on whether the follow-up for an individual ends with an observed event or for any other reason. We used 1/10 of a year as the length of each time interval, which gave approximately 343,000 observations on the dataset that held the data for analysis.
This approach was used, as risk factors that change during follow-up are automatically updated and corrected for, as age is used as a time-dependent risk factor.
All tests were two-tailed and the level of significance was set at P ˂ .05. Statistical analyses were performed with SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
At the first examination, 80 participants (53 men and 27 women) were diagnosed with AF (3.0% of the study population). During 30 years of follow-up, another 478 participants (18.8%), 203 men and 275 women, were diagnosed with AF. shows the number of new subjects diagnosed with AF in the three cohorts between 70 and 100 years. The majority of AF cases (59%) were first identified through the NPR, 39% were diagnosed at screening examinations and 2% of the AF were first diagnosed through the Cause of Death Register alone.
Table 1. Number of participants with atrial fibrillation diagnosed between the age of 70 and 100 years, in subjects without atrial fibrillation at baseline, by method of first detection in three population-based cohorts, all 70 years of age at baseline.
The prevalence of AF by age is shown in . Among men, 4.4% of the population were diagnosed with AF at the age of 70 years. The prevalence was 14.4% at the age of 80 and 16.8% at the age of 90. The corresponding figures for women were 1.9%, 8.7% and 21.2%, respectively. Separate tests in 1-year intervals of age showed a significantly higher prevalence among men compared with women up to the age of 83. After this, no significant gender differences were observed.
Figure 1. Prevalence of atrial fibrillation at ages 70–100 years in three population-based cohorts of men and women.
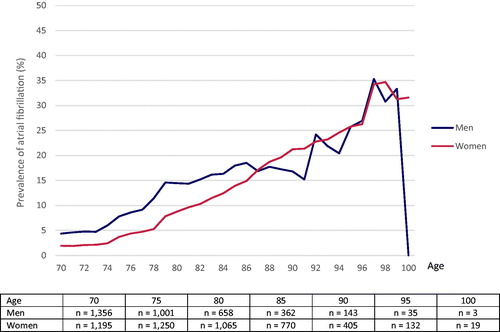
The overall incidence of AF during follow-up among men and women 70–99 years of age was 14.9 per 1,000 person-years at risk. shows the incidence rates in 5-year intervals for each cohort among men and women. The incidence rate increased from 5.1 per 1,000 person-years at risk in men and 1.9 per 1,000 person-years at risk in women aged 70–74, to 46.7 per 1,000 person-years at risk in men and 47.1 per 1,000 person-years at risk in women aged 90-94. In the age interval of 95 to 99, the incidence rate per 1,000 person-years at risk was 93.3 in men and 56.6 in women, although the confidence intervals (CIs) were very wide.
Table 2. Incidence rate of atrial fibrillation per 1000 person-years at risk for men and women and in three different population-based cohorts followed from 70 to 100 years of age.
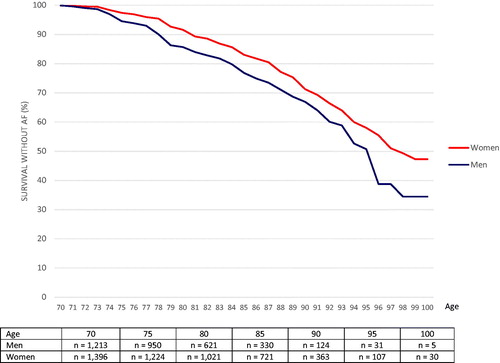
shows AF-free survival between 70 and 100 years of age for all participants (all three cohorts). The risk of AF was significantly higher among men compared with women, RR: 1.43 (95% CI: 1.19–1.72). The cumulative incidence of AF from 70 to 100 years of age was 65.6% for men and 52.8% for women.
Figure 2. Cumulative incidence of men and women surviving without being diagnosed with atrial fibrillation between the age of 70 and 100 years.
In a multivariate Poisson regression model including sex, age, smoking, hypertension, serum cholesterol, BMI and diabetes at baseline examination, participants who developed AF ran a higher risk of death. As seen in , both men and women with AF had a significantly higher mortality rate compared with men and women without AF, RR: 2.05 (95% CI: 1.74–2.40) for men and RR: 1.82 (95% CI: 1.58–2.10) in women, p < 0.0001. There was no significant difference in the impact of AF on mortality related to age or sex (test of interaction between AF and mortality in the total model related to sex: RR = 0.987 p = .87; related to age: RR = 1.107, p = .11).
Table 3. Atrial fibrillation as a predictor of death at different age intervals.
The RR for mortality related to AF did not differ between the three cohorts (data not shown). A subgroup analysis was performed including only participants from Cohort 1 where AF was first registered on an ECG at any of the screening examinations (n = 91), excluding AF first diagnosed from hospital records and death certificates. This revealed a considerably lower RR for death among participants with AF: 1.29 (95% CI: 1.03–1.63). The analysis was made with an identical Poisson regression model with the same covariates as in .
Discussion
This study of three representative samples from the general population showed a cumulative incidence of AF, from 70 to 99 years of age, of 65.6% for men and 52.8% for women.
As shown in other studies [Citation1,Citation4,Citation26,Citation27], the prevalence and incidence rate of AF increases markedly with age, and the prevalence peak is almost 30% at the age of 90 years and above, which is consistent with data from our study. However, few previous studies have included subjects in the oldest age groups and followed them longitudinally over many years.
In this study, the prevalence of AF was significantly higher among men compared with women up to the age of 83 but not thereafter. The Rotterdam study showed no sex-difference in prevalence of AF after the age of 80 years [Citation27], while Björck et al. [Citation1] showed approximately 5% higher prevalence among men compared to women.
Our data focusing on people at age 70 or older and including screening as well as hospital data showed that a large proportion of the general population could expect to develop AF if they survived to 100 years of age. Even with this substantial proportion eventually developing AF, it is likely that we are underestimating the prevalence in our study. When we compare Cohort 1 with Cohorts 2 and 3, we see more ECG-verified cases in Cohort 1 compared with the other two groups. This can be explained by the frequent screening examinations performed in this cohort up to the age of 100 years. The NPR only includes hospitalised in-patients diagnosed with AF and many older patients with sole or uncomplicated AF are never admitted to hospital.
Screening examinations were only performed up to age 79 years in Cohort 2 and up to age 86 years in Cohort 3. After this, all the data on AF were collected from the NPR. The fact that we found a large proportion of AF at screening that was not reported in the NPR is in accordance with an earlier study reporting an underestimation of prevalence of at least 20% if only analysing NPR cases [Citation28]. On the other hand, the incidence of NPR-diagnosed AF may have been slightly underestimated in Cohort 1, as we are lacking some data from the NPR before age 77 years for this cohort.
Newer screening methods with intermittent two-week ECG recordings have shown almost double the prevalence of AF compared with previously performed single ECG studies [Citation12,Citation29]. This suggests that many cases of paroxysmal AF remain undiagnosed.
The incidence rate increases with age, as has previously been described [Citation26] but it has been reported to stabilise after the age of 85 [Citation27]. We observed a continuous increase among the very old, with an incidence rate of 46.6 per 1,000 person-years at risk for men and 47.1 for women at age 95 years. Two hundred and fifty-one women and 61 men were followed for another 5 years after age 95.
Although the sample size in the highest age group was limited, the number of elderly men and women in our study was higher than in previous studies, showing a lifetime risk at age 80 of about 20%. Our data suggest a considerable increase in the prevalence of AF with the increasing number of very old people in the population.
The increasing risk of AF with age could be explained by age itself. Furthermore, morphological and electrophysiological changes may occur in older age, as there is an increase in connective tissue with secondary effects on atrial compliance, increased atrial pressures and electrophysiological dispersion [Citation30–32].
There is consensus that AF is a risk factor for mortality, even though the causality and the true power of AF compared with associated risk factors have been discussed [Citation33]. Several studies have found an approximate doubling of the mortality risk among patients with AF compared with controls free from AF [Citation6,Citation17–19], consistent with our data from all three cohorts. Irrespective of age, sex, hypertension, diabetes, smoking, BMI and cholesterol levels, AF was associated with a significant over-mortality throughout the observation period, but not to the same extent when AF diagnosed by hospital registers was excluded. In the subgroup analysis of participants from Cohort 1 examined frequently over the whole 30-year period, excluding patient registry data, we found that, the RR for mortality was lower among participants with AF first diagnosed at ECG during a screening procedure than for the whole group of participants with AF. The excess risk of death thus appears to be lower than previously reported among older people with AF diagnosed at out-patient screening examinations. Some more recent studies support our finding in the subgroup analysis, of a lower mortality risk in AF [Citation18,Citation19,Citation34]. Andersson et al. [Citation19] showed no increased mortality rate among men with lone AF at ages of 75 years and above, and mortality rates were consistent with our data at ages between 65 and 74 years. The same study showed a hazard ratio for mortality of 1.4 in women, with AF, at the age of 65 and above.
Data collected from NPRs may overestimate mortality rates, where hospitalised patients are likely to obtain their AF diagnosis due to more serious conditions such as myocardial infarction, heart failure or stroke [Citation35]. Hospital admissions are also more prevalent in patients with comorbidities, which will in turn influence the risk of death.
Some previous studies have suggested a decreasing relative risk of death from AF with age [Citation6,Citation19]. In this study, this was not verified, even though there was a non-significant trend towards a lower RR for death among participants with AF with increasing age. Earlier studies have also shown a substantially higher risk of death in women compared with men with AF [Citation18]. No clear evidence of interaction was found in this study, which might be due to lack of sufficient power.
The finding that participants with AF diagnosed at screening examinations, had a considerably lower risk of death compared to subjects first diagnosed from hospital records, may have clinical implications. Screening for AF is becoming more accessible through new techniques [Citation36]. However, the knowledge of long term prognosis for asymptomatic patients with diagnoses of AF at screening is still very limited, and more studies with long term follow-up of patients diagnosed with AF though screening procedures are needed.
Improved preventive care and better treatment may lead to a lower rate of hospitalisation among patients with AF in the future. However, it is likely that a larger proportion of the population will be affected by AF, as average life expectancy increases. These findings justify the further optimisation of prevention and treatment through life, in order to reduce mortality and morbidity, especially since AF is associated with significant costs [Citation37–39].
Strengths and limitations
The main strength is the large representative population samples of men and women followed over a period of 30 years, with repeated screening examinations, and the detailed follow-up.
One limitation is the fact that the study is fairly old and the treatment of other conditions such as myocardial infarction, stroke, heart failure and hypertension has improved. Moreover, survival among patients with AF may be better today [Citation40–44]. At the time of the study, the use of oral anticoagulation therapy among older patients was low. This can be regarded as both a limitation and a strength, as it may better describe the true impact of AF on mortality.
The method of using single 12-lead ECG recordings might contribute to an underestimation of the incidence and prevalence of AF, as AF is paroxysmal in many instances.
Conclusion
Among persons surviving to the age of 70, the cumulative incidence of AF was over 50% during follow-up to age 100, both among men and women. Participants with AF had double the mortality risk compared with men and women without AF. Participants with AF first recorded at a screening examination only had a 29% increase in mortality compared with participants without AF.
Author contributions
GL, ZM, BL and POH contributed to the conception or design of the work. GL drafted the manuscript. All authors contributed to the acquisition, analysis or interpretation of the data. All authors critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bjorck S, Palaszewski B, Friberg L, et al. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44:3103–3108.
- Mandalenakis Z, Von Koch L, Eriksson H, et al. The risk of atrial fibrillation in the general male population: a lifetime follow-up of 50-year-old men. Europace. 2015;17(7):1018–1022.
- Andersson P, Londahl M, Abdon NJ, et al. The prevalence of atrial fibrillation in a geographically well-defined population in northern Sweden: implications for anticoagulation prophylaxis. J Intern Med. 2012;272(2):170–176.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046.
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364.
- Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34(14):1061–1067.
- Lakshminarayan K, Anderson DC, Herzog CA, et al. Clinical epidemiology of atrial fibrillation and related cerebrovascular events in the United States. Neurologist. 2008;14(3):143–150.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
- Henriksson KM, Farahmand B, Asberg S, et al. Comparison of cardiovascular risk factors and survival in patients with ischemic or hemorrhagic stroke. Int J Stroke. 2012;7(4):276–281.
- Boriani G, Laroche C, Diemberger I, et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med. 2015;128(5):509–518 e2.
- Flaker GC, Belew K, Beckman K, et al. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(4):657–663.
- Mandalenakis Z, Lennartsson ST, Fu M, et al. The incidence of atrial fibrillation and the added value of thumb ECG for detecting new cases. Scand Cardiovasc J. 2018;52:256–261.
- Doliwa PS, Rosenqvist M, Frykman V. Paroxysmal atrial fibrillation with silent episodes: intermittent versus continuous monitoring. Scand Cardiovasc J. 2012;46(3):144–148.
- Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–484.
- Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844.
- Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40(1):84–92.
- Marijon E, Le Heuzey JY, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–2201.
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952.
- Andersson T, Magnuson A, Bryngelsson IL, et al. Gender-related differences in risk of cardiovascular morbidity and all-cause mortality in patients hospitalized with incident atrial fibrillation without concomitant diseases: a nationwide cohort study of 9519 patients. Int J Cardiol. 2014;177(1):91–99.
- Chapa DW, Akintade B, Thomas SA, et al. Gender differences in stroke, mortality, and hospitalization among patients with atrial fibrillation: a systematic review. Heart Lung. 2015;44(3):189–198.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847.
- Rinder L, Roupe S, Steen B, et al. Seventy-year-old people in Gothenburg: a population study in an industrialized Swedish city. Acta Med Scand. 2009;198(1–6):397–407.
- Nilsson LV, Persson G. Prevalence of mental disorders in an urban sample examined at 70, 75 and 79 years of age. Acta Psychiatr Scand. 1984;69(6):519–527.
- Lernfelt B, Forsberg M, Blomstrand C, et al. Cerebral atherosclerosis as predictor of stroke and mortality in representative elderly population. Stroke. 2002;33(1):224–229.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450.
- Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–378.
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953.
- Baturova MA, Lindgren A, Carlson J, et al. Atrial fibrillation in patients with ischaemic stroke in the Swedish national patient registers: How much do we miss?. Europace. 2014;16(12):1714–1719.
- Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131(25):2176–2184.
- Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103(5):769–777.
- Ferrari R, Bertini M, Blomstrom-Lundqvist C, et al. An update on atrial fibrillation in 2014: from pathophysiology to treatment. Int J Cardiol. 2016;203:22–29.
- Wasmer K, Eckardt L, Breithardt G. Predisposing factors for atrial fibrillation in the elderly. J Geriatr Cardiol. 2017;14(3):179–184.
- Leong DP, Eikelboom JW, Healey JS, et al. Atrial fibrillation is associated with increased mortality: causation or association? Eur Heart J. 2013;34(14):1027–1030.
- Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591–1602.
- Panaccio MP, Cummins G, Wentworth C, et al. A common data model to assess cardiovascular hospitalization and mortality in atrial fibrillation patients using administrative claims and medical records. Clin Epidemiol. 2015;7:77–90.
- Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917.
- Ericson L, Bergfeldt L, Bjorholt I. Atrial fibrillation: the cost of illness in Sweden. Eur J Health Econ. 2011;12(5):479–487.
- Aronsson M, Svennberg E, Rosenqvist M, et al. Designing an optimal screening program for unknown atrial fibrillation: a cost-effectiveness analysis. Europace. 2017;19(10):1650–1656.
- Delaney JA, Yin X, Fontes JD, et al. Hospital and clinical care costs associated with atrial fibrillation for Medicare beneficiaries in the Cardiovascular Health Study and the Framingham Heart Study. SAGE Open Med. 2018;6:2050312118759444.
- Vaartjes I, O’Flaherty M, Capewell S, et al. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44(3):591–597.
- Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–353.
- Coles AH, Fisher K, Darling C, et al. Long-term survival for patients with acute decompensated heart failure according to ejection fraction findings. Am J Cardiol. 2014;114(6):862–868.
- MacIntyre K, Capewell S, Stewart S, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation. 2000;102(10):1126–1131.
- Krumholz HM, Wang Y, Chen J, et al. Reduction in acute myocardial infarction mortality in the United States: risk-standardized mortality rates from 1995–2006. JAMA. 2009;302(7):767–773.
