Abstract
Objectives. We present the outcome of the first 80 patients receiving a continuous flow left ventricular assist device at Helsinki University Hospital between December 2011 and November 2018. Design. This was a single-center retrospective study. We describe our patient management in detail. The primary end-points were death, heart transplantation, or pump explant. Data was reported in accordance with the Interagency Registry for Mechanical Circulatory Support protocol. All patients receiving an assist device during the study period were included in the data analysis. Results. Mean patient age was 53 ± 12 years at implantation and 85% were male. Most patients suffered from dilated (48%), or ischemic (40%) cardiomyopathy. One-third of patients were bridged with venoarterial extracorporeal membrane oxygenation to assist device implantation. Implant strategy was bridge to transplant or bridge to decision in most patients (88%). Mean follow-up time on pump was 529 ± 467 days. Survival was 98, 92, 85, 79 and 71% at 1, 3, 12, 24 and 36 months, respectively. Most common causes of death were multi-organ failure, right heart failure, or stroke. Only three patients (4%) had suspected pump thrombosis, two of which resolved with medical treatment and one resulting in death. Pump exchange or explant were not performed in a single patient. Neurological events occurred in 18%, non-disabling stroke in 8%, and fatal stroke in 4% of the patients. The incidence of device-related infection was 10%. Conclusions. Survival rates were good, although one third of patients were bridged with temporary circulatory support. We report a high level of freedom from pump thrombosis, fatal stroke, and driveline infection.
Introduction
Heart transplantation (HTx) and durable mechanical circulatory support (MCS) are two effective treatment options for patients suffering from end stage heart failure (HF) refractory to medical or other therapy. HTx has been the golden standard, offering better survival and quality of life [Citation1]. The use of durable MCS with continuous flow left ventricle assist devices (CF LVAD) in treating advanced HF has been steadily growing due to limited availability of donor organs. Globally, LVAD therapy is increasingly being used for destination therapy (DT) [Citation2].
The use of CF LVAD may be associated with several major complications. Neurological events are reported to occur in 17-38% of patients and stroke in 10 -30%. In recent randomized large trials on CF LVAD, 10% of patients had previous stroke, and 16% − 19% had a history of transient ischemic attack [Citation3,Citation4]. The risk of pump thrombosis with CF LVADs is low but a high level of anticoagulation and antithrombotic treatment is necessary. Together with acquired coagulopathy, this increases the likelihood of bleeding. Driveline infections are associated with worse outcome and occur in 15 -24% % of patients. Functional class and survival are however significantly better in advanced HF patients on CF LVAD than patients on medical therapy [Citation3–6].
At present, HTx seems to be the better option for treating end-stage HF. CF LVAD therapy in bridging patients to transplantation is reasonable and effective. In order to achieve comparable results also in DT patients, an improvement in long-term survival and reduction in complication rates must be demonstrated.
In this study, we report our single center results of CF LVAD use in Finland between 2011 and 2018.
Methods
Study design
This single-center study was conducted at Helsinki University Hospital, the only heart transplantation and ventricular assist device-implanting center in Finland. Data was collected retrospectively and the primary end-points were death, HTx, or LVAD explant. Causes of death and adverse events were investigated by review of electronic medical records of the patients and defined according to the Interagency Registry for Mechanical Circulatory Support (INTERMACS) manual of operations. The institutional review board, Helsinki University Hospital approved all data collection and analysis.
Study patients
Between December 2011 and November 2018, 80 patients were implanted with a CF LVAD, HVAD (HeartWare Inc., Framingham, MA, USA), which was the only available durable MCS during the study period in Finland. All patients were included in the study and followed through the end of January 2019.
Patient selection and management
Patients with end-stage HF refractory to optimal medical or other, medical device treatment were considered for LVAD implantation by our heart team consisting of a cardiac surgeon, cardiologist, and cardiac anesthesiologist. Other specialists were consulted as needed. Treatment strategy at implantation was bridge to decision (BTD), bridge to transplantation (BTT), DT, or rescue therapy. Patients were bridged due to critical illness, continuous deterioration or due to a reversible contraindication for direct transplantation such as pulmonary hypertension. Destination therapy was offered to elderly patients.
Our strategy to prevent perioperative right heart failure included preoperative reduction of fluid overload pharmacologically or by dialysis, intraoperative fluid ultrafiltration, and intravenous inotropic treatment usually with milrinone initiated at least 24 h prior to LVAD implantation. Levosimendan was used in frequent flyers and some acute patients before bridging with ECMO but was not routinely administered preoperatively.
Implantations were performed by dedicated surgeons via a full sternotomy with the use of cardiopulmonary bypass and ventricular fibrillation without aortic cross clamping. Fibrillation offers better visualization of the LV cavity and inflow cannula tract due to less bleeding and motion of the heart. The outflow graft was directed to the right and sewn to the ascending aorta by tangential aortic clamping. The driveline was tunneled between the abdominal muscles and the posterior muscle fascia. An exit wound was created at the level of the umbilicus on the right. The velour-covered part of the driveline was left several centimeters subcutaneously. LVAD speed was gradually increased during weaning from bypass and the final speed (usually 2400–2700 rpm) was decided based on echocardiography findings, with the goal to have the interventricular septum close to the midline. Post-operatively patients were monitored with a pulmonary artery catheter and the HVAD monitor to ensure adequate RV function and CF LVAD flow.
At present, all patients are postoperatively treated with milrinone, isoprenaline and inhaled nitric oxide, which are gradually weaned at the intensive care unit. Sildenafil treatment is started during weaning of nitric oxide. As soon as the vasopressors are weaned, heart failure medications are started including beta-blockers, ACE-inhibitors, loop diuretics and an aldosterone antagonist.
Heparin-infusion was started 24 h after surgery with a target activated partial thromboplastin time (APTT) of 40–50 s, which was increased to 50–60 s on the second postoperative day, and continued until INR had been maintained within the therapeutic range for two days. Warfarin was started on the first postoperative day. A target INR of 2.5–3 was most commonly used but could be modified based on the status of the patient. Daily vitamin K supplementation was given to all patients in order to stabilize INR values. Aspirin was started during the implant hospitalization after removal of all chest tubes. The initial Aspirin dose was 100 mg and later adjusted based on patient status and platelet function tests with Multiplate®. Non-responders to Aspirin were switched to Clopidogrel. Patients routinely received low-dose simvastatin (10–20 mg) therapy for endothelial protection.
Aspirin medication was stopped in patients with recurrent gastrointestinal bleeding. The INR target was lowered to 1.8–2.5. In bleeders, endoscopy of the gastrointestinal tract was performed at least once, including esophagogastroduodenoscopy and colonoscopy. Capsule-endoscopy or double-balloon enteroscopy was used when necessary. Propranolol therapy was started in patients with angiodysplastic bowel lesions. Proton pump inhibitors were routinely used by all LVAD patients. Ocreotide therapy with daily subcutaneous injections was used in some cases and bevasitsumab therapy in an extremely challenging bleeder. Patients were also screened for iron-deficiency and received intravenous iron when necessary.
Postoperative antibiotic prophylaxis consisted of meropenem and vancomycin, which was stopped after removal of chest tubes. All patients received permanent trimethoprim/sulfamethoxazole or doxycycline prophylaxis once daily to prevent driveline infections.
After the implant hospitalization, all patients were followed by our LVAD coordinator and at our LVAD outpatient clinic every 1–3 months. Laboratory tests were performed every 1–3 weeks, including INR and hemolysis markers. Patients with INR values below the therapeutic range received low molecular weight heparin (LMWH). The driveline exit wound was inspected regularly and kept covered with sterile wound dressings or a chlorhexidine gluconate patch. The wound was treated by the patients and rinsed with normal tap water. Bacterial cultures were taken if infection was clinically suspected and treated accordingly. Hypertension was medicated to achieve a mean arterial pressure of 70–80 mmHg. For BTT patients, cardiac catheterization and other evaluation was performed, usually 3–6 months after implantation to determine right heart function and pulmonary vascular resistance. All patients were evaluated and treated by our physical therapist and by a nutritional therapist if necessary. Initial evaluation of some acute complications were performed in other participating hospitals. All patients with major complications were transferred to and managed at Helsinki University Hospital. Patients and local primary caregivers were encouraged to contact our LVAD coordinator or surgeon on call in cases of emergency. During the implant hospitalization, all patients were trained to follow their CF LVAD parameters, take care of the driveline wound and monitor clinical parameters such as weight and swellings.
Statistical methods
All statistical analyses were performed using SPSS 22 (IBMVR SPSSVR Statistics, version 22). Categorical variables are reported in frequencies and continuous variables as mean ± standard deviation with range. Kaplan–Maier plots were used for survival analysis and the Log Rank test for determining significance. Fischer’s exact test was used for contingency analysis. A p-value of less than .05 was considered significant in all analyses.
Results
Baseline characteristics
Detailed baseline characteristics of the study cohort are presented in . Mean age was 53 ± 12 years (17–69 years). Patients were mostly male (85%) and suffered predominately from dilated (48%) or ischemic (40%) cardiomyopathy. Most of the patients were in INTERMACS class 2 or 3 (78%), and about one-third of patients (33%) were bridged with ECMO to LVAD. Ten patients (13%) had a previous stroke. Only nine devices (11%) were implanted for destination therapy. Two patients had concomitant tricuspid valve repair.
Table 1. Baseline characteristics of patients receiving a CF LVAD.
Outcomes
After surgery, the patients required mechanical ventilation for 3.4 ± 6.9 days (range 1–49) and intravenous inotrope therapy for 6.8 ± 4.2 days (range 1–20). Mean length of ICU stay was 10.8 ± 9.1 days (range 1–52) and mean length of hospital stay was 34.3 ± 17.9 days (range 10–103). Patients on preoperative ECMO required longer hospital stays than the ones without. However, the difference was not significant (40 ± 21 days vs. 32 ± 16 days, p=.11). Seventy-three patients (91%) were discharged home, one patient (1%) to another hospital and 6 (8%) patients died during the implant hospitalization ().
Table 2. Postoperative course after CF LVAD implantation.
Nine patients (11%) were re-operated within 48 h due to bleeding, and two patients (3%) required reoperation due to aortic dissection. Both aortic dissection patients had intra-aortic balloon pump (IABP) prior to LVAD implantation. Both patients were treated with endovascular repair of the descending thoracic aorta. One of them also required reconstruction of the ascending aorta and proximal arch.
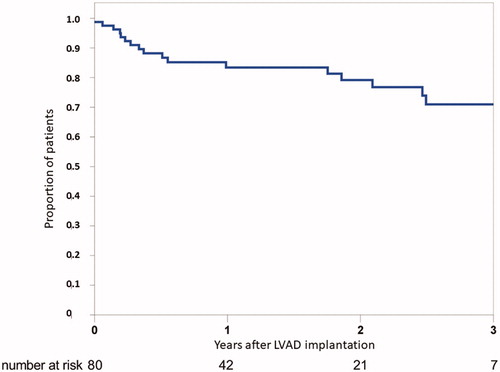
Survival after LVAD implantation is presented in . Mean follow-up was 752 ± 654 days (range 1–2618) and time on LVAD was 529 ± 467 days (range 1–2254) (). Survival was 98, 92, 85, 79 and 71% at 1, 3, 12, 24, and 36 months, respectively. We did not observe any statistically significant effect of gender, disease, strategy, or preoperative ECMO-treatment on survival with Kaplan-Meier analysis. Younger patients seemed to do better but the difference remained statistically insignificant (). In the whole cohort, 28 patients (36%) have been transplanted out of which two thereafter died. One heart transplant recipient died due to primary graft dysfunction and the other due to mesothelioma 4 years after transplantation. Time to transplantation was 663 ± 463 days (range 143–2247). Eighteen (23%) died while on LVAD, and the mean time to death was 398 ± 436 days (range 1–1470). Thirty-four (43%) patients currently have a pump with an average follow-up of 484 ± 463 days (range 75–2254).
Figure 2. Kaplan–Meier survival analysis for age (a), diagnosis (b) and strategy (c). Differences between groups were insignificant with Log-Rank tests. BTT/BTDl: bridge do transplantation/decision; DT: destination therapy.
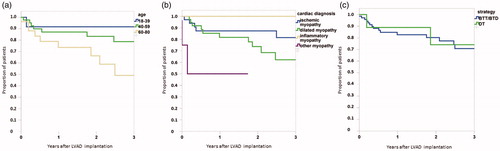
Table 3. Outcomes and causes of death after CF LVAD implantation.
Causes of death are presented in . The most common cause of death was multi-organ failure (MOF) (4), followed by right heart failure (RHF) (3), stroke (3), amiodarone-induced acute respiratory distress syndrome (ARDS) (2), and sepsis (2). One patient died from pump thrombosis and consequent heart failure after initial successful thrombolysis. Other causes consisted of suicide (1), anaphylaxis (1) and device stop due to accidental removal of all power sources (1). Two of the patients died during the first 30 days after implantation. The first patient was on ECMO due to postcardiotomy cardiogenic shock and underwent LVAD implantation in MOF as the last attempt to rescue him, but he died in fulminant liver failure and hemodynamic collapse within a few hours after surgery. The second patient with multiple drug hypersensitivity was readmitted to hospital, given intravenous antibiotics, and died due to anaphylactic shock on day 22 after LVAD implantation.
Adverse events
Two patients required temporary RVAD support at the time of LVAD implantation. Additionally, severe RHF was observed in 13 other patients (16%) on average 95 ± 142 days (range 2–483) after implantation (). Nine patients with RHF suffered from dilated cardiomyopathy and four patients from ischemic cardiomyopathy. Statistically, there was no difference between the two groups (p=.23). In 10 patients, RHF resolved with conservative treatment, including intravenous diuretics, ultrafiltration, and milrinone infusion. Three patients (4%) developed severe right heart failure leading to MOF and death. Fourteen patients (18%) had respiratory failure leading to prolonged mechanical ventilation and death in two patients (3%). Both deaths were associated with amiodarone-induced ARDS.
Table 4. Adverse events after CF LVAD implantation.
There was 14 patients (18%) with neurological events that occurred 320 ± 361 days (range 24–1230) after implantation. Three patients (4%) suffered from fatal stroke; two of them had previous strokes. Six more patients (8%) suffered from milder strokes with 3 or less points on the modified Rankin scale. Two patients were invasively treated with interventional embolectomy and thrombus aspiration. The other stroke patients were treated conservatively. Four patients (5%) suffered from transient ischemic attacks. One patient (1%) had a non-disabling subarachnoid bleeding. One more patient (1%) had a non-disabling spontaneous intracerebral hemorrhage. Peripheral embolism to the upper extremity was observed in one patient (1%) and was successfully treated with embolectomy ().
We did not perform a single pump exchange during the study period and observed only three cases of suspected pump thrombosis (4%) diagnosed by increase in plasma lactate dehydrogenase and abnormal pump parameters. One asymptomatic patient was successfully treated with heparin infusion. Two patients required thrombolysis therapy. One died after incomplete thrombolysis and re-thrombosis. The patient was not considered for urgent transplantation or pump exchange due to poor overall condition. The other patient was transplanted shortly after successful thrombolysis (). One patient (1%) suffered from hemolysis without changes in pump parameters and was managed with intravenous heparin.
We observed pump malfunction in four patients. A destination therapy patient accidentally cut the driveline with scissors and the pump was stopped for 4 h. The driveline was repaired and the patient was discharged home without any complications. Controllers were exchanged in three patients (4%) due to minor malfunction without compromise of circulatory support.
Driveline exit-wound infection was observed in 7 patients (9%). One patient had a deep driveline infection (1%). Mean time to the first driveline infection was 493 ± 499 days (range 52–1306). Species causing driveline infections were Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, Stenotrophomonas maltophilia, Moraxella species, and Pseudomonas aeruginosa. Three patients (4%) had driveline infections with positive blood cultures. One patient (1%) died from sepsis caused by driveline infection. Non-pump related causes of major infection and sepsis were more frequent. Thirty-four major infection events was observed in the study population ().
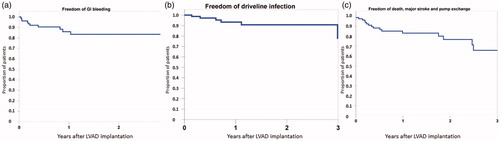
Early postoperative bleeding requiring surgery occurred in 9 patients (11%). Major bleeding later than 48 h after LVAD implantation occurred in 20% of the patients. Mean time to first bleeding was 61 ± 91 days (range 6–378). Gastrointestinal bleeding was diagnosed in 11 patients (14%). Other bleedings were mediastinal (6%) or pleural (1%). Thirty-two major bleeding events were documented; none of them was fatal (). demonstrates freedom from bleeding, infection and the composite outcome of death, disabling stroke or LVAD explant.
Figure 3. Kaplan–Meier analysis of freedom of gastrointestinal bleeding (a), driveline infection (b) and composite of death, major stroke or pump exchange (c).
After discharge home, 40 patients needed to be readmitted (50%). Mean time to the first readmission was 214 ± 252 days (range 22–1230). Total number of readmissions was 97. Twenty patients needed only one readmission, 7 patients were re-hospitalized twice and 13 patients required more than two readmissions. Most common causes of readmission were non-pump related infection (Citation19), gastrointestinal bleeding (23), right heart failure (Citation13), and stroke (Citation11). Pump malfunction requiring speed re-adjustment or controller exchange (n = 9), driveline infection (n = 8), and arrhythmia (n = 4) were less frequent. Twelve other readmissions were due to other infrequent causes.
Functional outcomes
Most of the patients suffered preoperatively from NYHA class IV symptoms (84%). Already 3 months after LVAD implantation patients functional class improved and at 1 year, most patients were in NYHA class I (73%), while few patients were in NYHA classes II (19%) and III (8%).
Baseline 6-minute walk test (6MWT) distances were extremely low 136 ± 184 m (range 0–570) due to a high percentage of patients on ECMO (33%) before implantation. Ambulatory patients’ 6MWT-result was 352 ± 112 m (range 160–570). During the first postoperative year 6MWT distance increased to 521 ± 120 m (range 227–710). Compared to age and sex-matched healthy subjects, the patients walked 75% of the reference distance at 3 months, 80% at 6 months and at later time points around 85% of the reference value.
Discussion
In this study, we present our single center experience of CF LVAD therapy in patients with end-stage heart failure. Most patients were bridged to transplantation. In the recent IMACS and INTERMACS reports, survival for all CF LVAD patients has been 81–83%, 71–73% and 62–62% at 1, 2 and 3 years [Citation2,Citation5]. In a follow-up report of the REVOLVE study, investigating long-term outcome in BTT HVAD patients, survival was 84, 78 and 68% during the first three years [Citation7]. Our survival rates are similar. Based on ISHLT reports regarding survival after heart transplantation performed after 2009, the survival rate at one year is 85% [Citation8]. This figure is also comparable to our results. Both heart transplantation and LVAD treatment offer significantly better outcome for end-stage heart failure patients than medical treatment alone [Citation6].
Most Finnish patients are treated as BTD or BTT. In Finland, during 2016–2018, HTx was performed in 5.2 patients per million population while CF LVAD was implanted in 3.4 patients per million population. Up to half of HTx, is currently performed from CF LVAD in Finland [Citation9]. The average waiting time for heart transplantation on LVAD was just under two years, which is similar to waiting times for our patients without MCS. In 2018, we observed a 10% mortality on our transplant waiting list (data available on www.scandiatransplant.org). About one third of our LVAD patients needed preoperative ECMO-support in cardiogenic shock. These patients would have died without LVAD implantation or high urgency heart transplantation. Therefore, LVAD implantation offered these patients the chance to receive a transplant. Additionally, the patients without need for ECMO support prior to LVAD implantation improved their physical condition during LVAD support, as evidenced by improved 6MWT results. The downside to transplanting patients on CF LVAD is the need for redo surgery and potential acquired bleeding disorders. We currently have no data on long-term outcome of HTx after LVAD therapy. However, a previous report has shown comparable results to HTx recipients without prior LVAD therapy [Citation10].
HTx patients in Finland have 1-year and 5-year survival rates of over 90 and 85%, respectively. The key in improving CF LVAD therapy is to extend the already achieved early survival benefit and improve long-term outcome, with the goal of achieving similar survival rates as for HTx [Citation8]. This might not be possible in elderly DT patients. Expanding DT strategy to younger patients is also controversial in a country like Finland since organ availability through the national and Scandiatransplant organizations are relatively good. We currently have an average survival after heart transplantation in Finland of approximately 14 years. One way to improve combined survival in BTT patients would be to increase bridging times and thereby extend cumulative survival. This approach was challenged by a recent report [Citation11] and still requires more data on long-term survival in our patients.
The leading cause of death in our study was MOF followed by RHF, stroke, ARDS and sepsis. Earlier studies with CF LVAD devices have reported lower RHF mortality [Citation1,Citation12] but in a recent report the incidence was 6% [Citation4]. Not surprisingly, patients with dilated cardiomyopathy had more episodes of RHF in this study. This difference was, however, not significant and was not related to cause of death. RHF and MOF is often a continuum, and the previous results could be related to differences in reporting since there is no general recommendation on how to report causes of death in MCS studies. In this study, most cases of RHF occurred more than 1 month after implantation. This could imply that these patients should have been managed more aggressively with LVAD speed adjustment, diuretics and fluid restriction.
In this study only 2 patients had concomitant tricuspid valve repair. This is done in our center when patients have severe tricuspid regurgitation, although there is no evidence for its long term benefits [Citation13].
Patients with heart failure often have several cardiovascular co-morbidities and are at risk for having strokes regardless of whether they are on CF LVAD or not, as seen by our preoperative 13% stroke incidence. During the first two years after implantation, stroke occurs in 10–30% of patients and disabling stroke in 1–7% [Citation1–3]. So far, infection and hypertension have most strongly been related to stroke risk [Citation14,Citation15]. These are factors we are trying to combat aggressively in our patients and report low numbers of both driveline infections and strokes. Although, we generally keep the INR target over 2.5, we have seen very few intracranial bleedings and our overall bleeding rate was relatively low. Fatal strokes were seen in only three patients. The patients with non-disabling strokes were rehabilitated effectively and evaluated for transplantation as early as possible. New strokes were not observed in these patients.
Bleeding, hemolysis and thrombosis are all related [Citation16,Citation17]. In the present study, we had very few cases of hemolysis and suspected pump thrombosis. This was achieved by keeping INR levels mainly over 2.5 and by performing platelet function tests to make sure patients responded to antiplatelet therapy. The use of these tests is supported by a recent report by Montalto et al. [Citation18]. The regular analysis of hemolysis markers allowed us to react to early signs of hemolysis. We treated all three cases of thrombosis medically and two of them successfully, although a recent meta-analysis showed clearly better survival in surgically treated patients [Citation19]. Major bleeding occurred in 20% and were mostly gastrointestinal. None of the bleedings were fatal and only one spontaneous ICH was life threatening. The patients that died after stroke all had hemorrhagic strokes and it is possible that our high level of antiplatelet and anticoagulant therapy increased this risk to some extent. Previous large CF LVAD trials have reported higher incidences of bleeding (28–60%) and pump thrombosis (1–14%) than in the present study [Citation1,Citation3,Citation4].
We report a low incidence of major device-related infection. We think this was achieved by careful patient monitoring and wound care together with prophylactic antibiotic therapy. Functional improvement of patients was seen early after hospital discharge and supports previous evidence of beneficial effects of CF LVAD treatment in terminal heart failure [Citation3,Citation4,Citation6].
This study has several limitations. It is a retrospective and single-center study. Data collection of end-points and adverse events was done in accordance with general guidelines by the authors but inter-observer bias of the data cannot be excluded. Some data might have been unavailable due to post-operative treatment of the patients in other hospitals. The study population followed for over two years was small and this could influence the results. Direct comparison to other trials can therefore not necessarily be made.
Conclusions
We show good survival rates of mainly BTT/BTD patients on CF LVAD, although one-third of patients were bridged with ECMO to LVAD. We report a low incidence of stroke, pump thrombosis and driveline infection. This was achieved through close surveillance of the patients and personal adjustment of anticoagulant and antiplatelet therapy and permanent infection prophylaxis. Attention was kept on controlling hypertension, active wound care and early signs of pump thrombosis.
Acknowledgements
LVAD coordinators Catharina Yesil, Marja-Liisa Hellstedt, Sini Puputti, Mira Määttä, Jenna Segerlöv and Liisa Pesonen are acknowledged for their valuable effort in the follow-up of our patients. Physical Therapist Linda Ulenius is acknowledged for her input on patient rehabilitation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schmitto J, Zimpfer D, Fiane AE, et al. Long-term support of patients receiving a left ventricular assist device for advanced heart failure: a follow-up analysis of the registry to evaluate the heartware left ventricular assist system. Eur J Cardiothorac Surg. 2016;50(5):834–838.
- Kirklin J, Xie R, Cowger J, et al. Second annual report from the ISHLT mechanically assisted circulatory support registry. J Heart Lung Transplant. 2018;37(6):685–691.
- Rogers J, Pagani FD, Tatoole AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451–460.
- Mehra MR, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device — final report. N Engl J Med. 2019;380(17):1618–1627.
- Kormos R, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Tranplant. 2019;38(2):114–126.
- Starling RC, Estep JD, Horstmanshof DA, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2-year results. JACC Heart Fail. 2017;5(7):518–527.
- Strueber M, Larbalestier R, Jansz P, et al. Results of the post-market registry to evaluate the heartware left ventricular assist system (ReVOLVE). J Heart Lung Transplant. 2014;33(5):486–491.
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013. Focus Theme: Age. J Heart Lung Transplant. 2013;32(10):951–964.
- Kiss J, Stark C, Lommi J, et al. The present and the future of heart transplantation in Finland. Duodecim. 2017;133(24):2413–2417.
- Yoshioka D, Li B, Takayama H, et al. Outcome of heart transplantation after bridge-to-transplant strategy using various mechanical circulatory support devices. Interactive Cardio Vasc Thorac Surg. 2017;25(6):918–924.
- Dardas TF, Cheng RK, Mahr C, et al. Adverse effects of delayed transplant listing among Patients with implantable left ventricular assist devices. J Card Fail. 2018;24(4):243–248.
- Krabatsch T, Netuka I, Schmitto JD, et al. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure – 1-year results from the Ce mark trial. J Cardiothorac Surg. 2017;12(1):23.
- Critsinelis A, Kurihara C, Kawabori M, et al. Outcomes in patients who underwent a concomitant tricuspid valve procedure during left ventricular assist device implantation. J Card Surg. 2019;34(12):1458–1464.
- Milano CA, Rogers JG, Tatooles AJ, et al. HVAD: The ENDURANCE supplemental trial. JACC HF. 2018;6(9):792–802.
- Cho SM, Moazami N, Katz S, et al. Stroke risk following infection in patients with continuous-flow left ventricular assist device. Neurocrit Care. 2019;31(1):72–80.
- Sieg AC, Moretz JD, Horn E, et al. Pharmacotherapeutic management of gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices. Pharmacotherapy. 2017;37(11):1432–1448.
- Patel SR, Vukelic S, Jorde UP. Bleeding in continuous flow left ventricular assist device recipients: an acquired vasculopathy? J Thorac Dis. 2016;8(10):E1321–E1327.
- Montalto A, Comisso M, Cammardella A, et al. Early aspirin nonresponders identification by routine use of aggregometry test in patients with left ventricle assist devices reduces the risk of pump thrombosis. Transplant Proc. 2019;51(9):2986–2990.
- Luc JGY, Tchantchaleishvili V, Phan K, et al. Medical therapy compared with surgical device exchange for left ventricular assist device thrombosis: a systematic review and meta-analysis. Asaio J. 2019;65(4):307–317.