Abstract
Objectives
Cardiac transplantation is an effective treatment for advanced heart disease and protection of the donor organ is directly associated with post-transplantation outcomes. Cardioplegic strategies intend to protect the donor heart against ischemic injury during transplantation procedures. In our study, the effects of three different cardioplegia solutions were evaluated in a rat heart donor model in terms of cellular base. Design. Cardioplegia solutions as St. Thomas, del Nido or Custodiol were administered to male Wistar albino rats until cardiac arrest. Arrested hearts were excised and incubated in cold cardioplegia solutions for 4 h. Organ bath experiments were performed using the right ventricular free wall strips of the heart tissues. ATP, sialic acid, TNF-α levels and MMP-9 activities were measured in heart tissues. Incubation media were also used to measure TNF-α and troponin-I levels following organ baths experiments. Results. Custodiol administration led to reduced myocardial contraction (p < .05), decreased ATP levels (p < .001) and increased both TNF-α levels (p < .05), and MMP-9 activity (p < .05). Additionally, troponin-I and TNF-α levels in media were significantly increased (p < .05), TNF-α levels were positively correlated with MMP-9 activities (r = .93, p = .007) and negatively correlated with ATP levels (r = −.91, p = .01) in the Custodiol group. Also, MMP-9 activities were negatively correlated with ATP levels (r = −.90, p = .01) Conclusion. Custodiol cardioplegia cannot prevent functional and cellular damage in donor heart tissue. St. Thomas or del Nido cardioplegia could result in superior functional and biochemical improvement during transplantation procedures. In this respect, these cardioplegic solutions may be more advantageous as cellular and functional.
Introduction
Heart failure is a pathological condition with a high mortality and morbidity rate. Cardiac transplantation is an important treatment that positively affects survival and improves the quality of life of patients. One of the most important factors that provide success at transplantation is the protection of the donor organ. Administration of or incubation in cold cardioplegic solution is a preferred protective method for the heart to be transplanted during the 4–6 h ischemia process [Citation1,Citation2].
Cardioplegic solutions provide a stable surgical area during cardiac surgery and transplantation. Another use of cardioplegic solutions is to create an immediate arrest and to preserve the tissue during an extended period of ischemia. Various solutions with different protective and chemical properties have been developed [Citation3–5]. Ion concentrations of crystalloid cardioplegic solutions are similar to the cytoplasm as low sodium (<1.6 g/L) and high potassium (>3.9 g/L). It has been shown that hyperkalemic crystalloid cardioplegia solutions keep the cell membrane in the depolarization phase of the action potential, leading to diastolic cardiac arrest [Citation6]. However, they may cause calcium entry into the cell and immediate vasoconstriction. Also, endothelial damage can occur following solution exposure because of the high potassium concentration. In addition, cardioplegia solutions with high sodium (>1.6 g/L) and low potassium (<0.8 g/L) concentrations increase vascular resistance and reduce endothelial damage [Citation7,Citation8].
Custodiol, St. Thomas and del Nido cardioplegia solutions are the solutions that have different ion contents commonly used in the clinics. Since the intracellular and extracellular action mechanisms of these solutions are not well known, it is not clear if they are sufficient to protect the donor organ. In our study, we compared the effects of Custodiol, St. Thomas and del Nido cardioplegia solutions on the rat donor heart. The functional responses to catecholamine and cellular injury processes were evaluated after 4 h exposure of cardioplegia. Tumour necrosis factor-alpha (TNF-α) (cytokine activation), matrix metalloprotease-9 (MMP-9) activity, sialic acid (SA) (extracellular remodeling), troponin-I (functional loss), adenosine triphosphate (ATP) (energy level) were measured.
Materials and methods
Ethical approval and animals
The current study was approved by the Local Ethics Committee on Animal Experiments of Istanbul University (442761). All experiments were performed according to ARRIVE guidelines, the U.K. animals Act 1986 and EU directive 2010/63/EU. The animals were provided from I.U. Aziz Sancar Research Institute of Experimental Medicine. In our study, male Wistar albino rats (n = 18) weighing 397 ± 7 g were used. All animals were housed in cages in a temperature of 24 ± 1 °C with a 12 h light/dark cycle and 50 ± 5% humidity and were provided standard laboratory diet and water ad libitum.
Surgical protocol and study groups
Animals were anaesthetized with intraperitoneal ketamine (70 mg/kg) and xylazine (10 mg/kg) mixture. Following sterile preparation of the chest, a median sternotomy was performed. Aorta was isolated and inferior vena cava was cut. Cold cardioplegia solution (Custodiol, del Nido or St. Thomas, approximately 10 mL) containing heparin (5 IU/g body weight) was infused into the aorta to wash the intracardiac vascular bed. Blood was removed via inferior vena cava. Hearts were excised after cardiac arrest and immediately placed into cardioplegia solution (+4 °C). Incubation was extended for 4 h in the cardioplegia solution of the related group of approximately 2–3 times the volume (10 mL). At the end of the fourth hour, incubation media was collected (T1) and used for biochemical measurements.
Rats were randomly divided into 3 groups: (1) Custodiol (CD) group; hearts were incubated for 4 h in Custodiol cardioplegia solution. (2) St. Thomas (ST) group; hearts were incubated for 4 h in St. Thomas cardioplegia solution. (3) del Nido (DN) group; hearts were incubated for 4 h in del Nido cardioplegia solution. The control group was not specifically designed because a solution with optimum safety for control conditions has not yet been developed. After incubation, muscle strips were removed from the right ventricular free wall to assess myocardial contractility. The rest parts of the ventricle not used in contractility measures and residual tissue were stored at −80 °C until the analyses.
Organ baths experiments
The muscle layer of the left ventricular wall provides a fault signal in evaluating the effect of drugs due to its thicker wall [Citation9]. Hence free wall of right ventricular strips (1 mm wide, 10 mm long and 0.5 mm thick) were placed in Krebs–Henseleit solution maintained 20 mL organ bath at 37 °C, pH 7.4, and gassed with 95% O2 to 5% CO2 following 4 h incubation. Krebs–Henseleit solution was prepared to be (mmol/L); 120 NaCl, 4.80 KCl, 1.25 CaCl2, 1.20 MgSO4, 1.20 KH2PO4, 25 NaHCO3 and 11 glucose. Ventricular specimens were allowed to stabilize for at least 30 min before drug challenge under a resting tension of 1 g. The effects on ventricular automaticity were tested in the spontaneously beating free wall of the right ventricle. For this, its atrial end was fixed to metallic support and the apical end was attached to a force transducer. No electrical stimulation was applied. Cumulative concentration-response curves to noradrenaline (10−8–3 × 10−5M) and isoproterenol (10−9–3 × 10−6M) in right ventricular strips were performed. Drugs were added to a 20 mL organ bath in volumes less than or equal to 0.1 mL. Recorded data frequency (beats min−1) and contraction force (g) were calculated as the average rate of the preparation recorded during the incubation for each drug concentration.
Biochemical evaluations
The supernatants of homogenated heart tissues in phosphate buffer solution (PBS:pH 7.4) were used for biochemical measurements.
Manual method: sialic acid
The concentration of sialic acid was expressed as nmol/mg protein. Briefly, 0.2 mL of the sample was mixed with 1.5 mL of 5% perchloric acid. Samples incubated in a water bath at 100 °C for 5 min were cooled and then centrifuged at 2500 g for 4 min. The supernatants were collected and 0.2 mL of Ehrlich’s reagent was added. After samples incubated at 100 °C for 15 min. At the end of the incubation, 1 mL of distilled water was added and measured at 525 nm with a spectrophotometer [Citation10]. Sialic acid concentrations were determined by the absorbance values obtained from the standard curve.
Enzyme-linked immunosorbent assays (ELISA) methods
Matrix metalloprotease-9 (MMP-9), tumour necrosis factor-α (TNF-α), adenosine triphosphate (ATP) and cardiac troponin-I were measured using the ELISA method in right ventricular tissue using a specific detection kit (Elabscience). Also, TNF-α and cardiac troponin-I levels were measured in incubation media at the end of 4 h (T1).
Statistical analysis
All data are presented as mean ± SD. One-way analysis of variance followed by the Tukey post-hoc test for multiple comparisons was used. The Mann–Whitney test was used in cases that did not comply with normal distribution for comparison between groups. Pearson correlation test was performed to establish the link between measurements. Data sets were analyzed using GraphPad Prism 5.0 statistical software (GraphPad Software, La Jolla, CA). A p < .05 was considered to be statistically significant.
Results
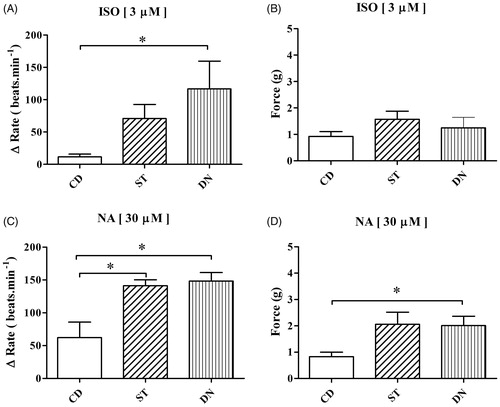
In the organ bath experiments, isoproterenol (3 µM) administration increased the ventricular beats in the DN group (117 ± 43 beats min−1) compared to the CD group (12 ± 4 beats.min−1, p < .05). In addition, there was no difference in the ST group compared to the other groups (p > .05, . Noradrenaline (30 µM) administration increased the ventricular beats in the DN group (148 ± 13 beats min−1) and ST group (141 ± 9 beats min−1) compared to the CD group (62 ± 24 beats min−1, p < .05). Also, the contraction force was higher in the DN group (2.3 ± 0.3 g) than in the CD group (0.8 ± 0.1 g, p < .05). The ST group was similar to the two groups (p > .05, ).
Figure 1. Effects of isoproterenol (ISO; 3 μM) on spontaneous ventricular rate changes (A) and contraction force (B) (*p < .05; compared to CD group). Effects of noradrenaline (NA; 30 μM) on spontaneous ventricular rate changes (C) and contraction force (D) (*p < .05; compared to CD group).
No differences in sialic acid levels were observed between right ventricular tissues in all groups in (p > .05). The mean sialic acid levels of ST, CD, and DN groups were 0.08 ± 0.01, 0.07 ± 0.01, 0.06 ± 0.01 nmol/mg protein, respectively ().
Figure 2. Right ventricular tissues sialic acid (SA) levels (A) in Custodiol (CD), St. Thomas (ST) and del Nido (DN) groups (p > .05; compared with all group). Right ventricular tissues MMP-9 activity (B) and TNF-α levels (C) in Custodiol (CD), St. Thomas (ST) and del Nido (DN) groups (**p < .01 and *p < .05; compared to CD group). Right ventricular tissues adenosine triphosphate (ATP) levels (D) in Custodiol (CD), St. Thomas (ST) and del Nido (DN) groups (***p < .001; compared to CD group).
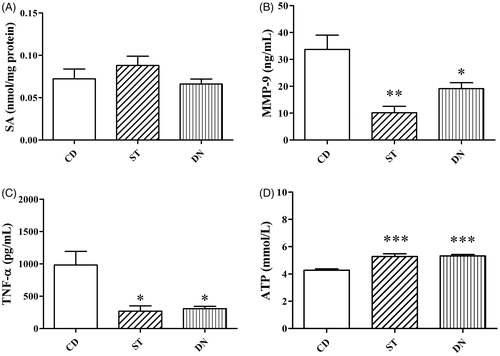
MMP-9 activities in right ventricular tissue were increased in the CD group (33.7 ± 5.3 ng/mL) compared to the ST (10.2 ± 2.4 ng/mL; p < .01) and DN groups (19.2 ± 2.2 ng/mL; p < .05, ). TNF-α levels in right ventricular tissue were increased in the CD group (985.8 ± 208.6 pg/mL) compared to the ST (269.8 ± 81.4 pg/mL; p < .01) and DN groups (306.7 ± 37.7 pg/mL; p < .05, ). No difference for both MMP-9 activities and TNF-α levels was found between the ST and DN groups (p > .05). (). ATP levels in right ventricular tissues were decreased in the CD group (4.3 ± 0.1 mmol/L) compared to the ST (5.3 ± 0.2 mmol/L; p < .001) and DN groups (5.3 ± 0.1 mmol/L; p < .001). No difference was found between the ST and DN groups (p > .05, ).
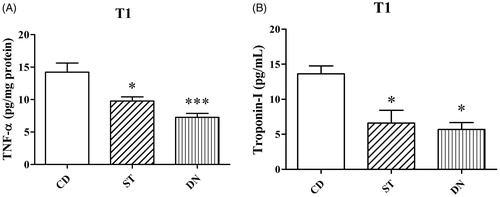
TNF-α levels of media at the end of experimentation were decreased in the ST (T1:9.8 ± 0.6 pg/mg protein) and DN groups (T1: 7.3 ± 0.6 pg/mg protein; p < .001) compared to the CD group (14.2 ± 1.4 pg/mg protein; p < .05, ). Additionally, troponin-I levels of media at the end of experimentation were decreased in the ST (T1: 6.6 ± 1.8 pg/mL) and DN groups (T1: 5.7 ± 1 pg/mL; p < .05) compared to CD group (T1: 13.6 ± 1.1 pg/mL; p < .05, ). No difference for both TNF-α and troponin-I was found between the ST and DN groups (p > .05, ).
Figure 3. TNF-α (A) and Troponin-I (B) levels in incubation (T1) solution after 4 h (***p < .001 and *p < .05; compared to CD group).
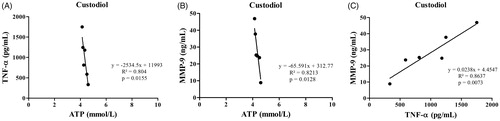
Tissue ATP levels were negatively correlated with TNF-α (r = −.91, p = .01, ) and MMP-9 (r = −.90, p = .01, ) activities in right ventricular tissue in the CD group. TNF-α levels in right ventricular tissues were positively correlated with tissue MMP-9 activities in CD group (r = .93, p = .007, ).
Discussion
The present study aimed at evaluating the effects of functionally and biochemically Custodiol, St. Thomas and del Nido cardioplegia in a rat model of the donor heart. For this purpose, a comparison of these three different cardioplegia solutions used in heart transplant surgery was performed for the first time. In our study, the inflammatory (TNF-α) and tissue remodelling (sialic acid, MMP-9) processes and contraction response against catecholamine in the organ bath were evaluated. Furthermore, tissue ATP contents and troponin-I levels were measured to determine tissue energy level and injury levels, respectively. The main findings of our study showed that: (1) Custodiol weakens the contraction effectiveness by decreasing tissue ATP content compared to St. Thomas and del Nido cardioplegia strategies. (2) The debilitating effect of Custodiol is the result of cellular damage as reflected by troponin-I levels. (3) Moreover, cellular damage has inflammation and tissue remodelling components.
A treatment method accepted approach for advanced heart failure is heart transplantion. To minimize the complications that may occur during transplantation, donor organ must be well protected during transplantation. Innovations in heart transplant surgery developed the first protective techniques against ischemia in the early 1960s and the cardioplegia solution developed by Bretschneider (Custodiol) revealed the protective effect of pharmacological arrest [Citation11]. Afterwards, cardioplegia solutions were renewed and developed to preserve the extracellular ion balance. In this line, the ion content of St. Thomas cardioplegia solution was prepared with potassium, calcium and magnesium at the appropriate concentration, consistent with plasma [Citation12]. As another one, del Nido cardioplegia solution, which is usually used with blood, was created in the 1980s to develop an effective cardioplegia as a single dose in paediatric cardiac surgery. The effect of del Nido cardioplegia, which has recently been used in adult surgery, on cellular processes has not been determined experimentally yet [Citation13,Citation14].
To provide cellular protection, cold cardioplegia is used to slow down the heart metabolic rate. The cold environment maintains ion content in the extracellular space and reduces the effect of harmful wastes [Citation8,Citation15,Citation16]. Despite the protective effect of hypothermia, cellular damage processes persistently proceed during extended ischemia. As oxidative phosphorylation is weakening due to hypothermia, energy stores being depleted. Thus, disruption and loss of myocardial function occur. Ischemia-induced increased oxidative species cause lipid peroxidation and activation of inflammatory mediators. Enzymes in the extracellular matrix are activated by oxidative mediators and participate in cell damage processes. Hence, it is important to evaluate these processes for the tissue to be transplanted [Citation17–19].
The contractile performance of the rat-right ventricle tissue was previously evaluated by applying vasoactive agents [Citation20]. In our study, we first evaluated the direct effects of cardioplegia solutions on the heart muscle with noradrenaline and isoproterenol and determined lower ventricular contraction force and rate in Custodiol group, compared to the St. Thomas and del Nido cardioplegia. As also shown in another experimental setup, Carmo et al. – in which Custodiol and del Nido (with blood) were compared – showed that dP/dtmax and coronary resistance levels were low in Custodiol administration after 3 h of ischemia [Citation21]. Also, it was presently shown that del Nido (without blood) application is more effective in terms of myocardial contractile strength. Therefore, it is suggested that Custodiol application may eventually lead to dysfunction of the heart tissue, and may be unresponsive to catecholamines. Moreover, high troponin-I release were also followed by a functional loss in Custodiol cardioplegia. High levels of troponin and protein expression in serum and tissue have already been associated with ischemia [Citation22]. These findings imply that the functional impairment that occurs after the application of Custodiol is due to cellular degradation.
In our study, low contractile strength also followed low ATP levels in Custodiol cardioplegia. Accordingly, glycolysis and ATP production are suppressed by both weak aerobic metabolism and the accumulation of hydrogen ions in the cell. Therefore, to maintain intracellular and extracellular ion balance and continue energy production, anaerobic glycolysis should be supported. St. Thomas and del Nido cardioplegia solutions contain sodium bicarbonate as a buffer solution to maintain intracellular pH and ion balance [Citation23]. The reason for the poor protective effect of Custodiol cardioplegia solution may be due to the lack of bicarbonate ion in it. Therefore, our findings suggest that Custodiol cardioplegia solution may have caused mitochondrial damage in the tissue. As a limitation, mitochondrial enzyme systems should be evaluated to support this result.
There are also studies linking the increase in TNF-α with loss of cellular function [Citation24]. TNF-α as an inflammatory mediator mediates the damage of chronic heart failure that contributes to heart remodelling. Increased levels of TNF-α in rat heart embryonic cells have been shown to increase protein expression of MMP-9 mRNA and pro-MMP-9 [Citation25]. This process has also been evaluated in different studies [Citation26]. Zheng et al. showed that increased MMP-9 activation after cardiac ischemia caused the destruction of ECM components and caused inflammation and tissue remodelling [Citation27]. In our study, the positive relationship between TNF-α level and MMP-9 activity revealed this process. Therefore, the inflammatory response caused by Custodiol changes the matrix by causing an increase in MMP-9 activity. The effects of cardioplegia solutions used in our study were also evaluated on cellular inflammation and myocardial tissue remodelling processes following 4-h ischemia. The adverse effect of Custodiol on myocardial function was followed by the inflammatory process as reflected by higher tissue TNF-α level. As well, inflammation was triggered by tissue remodelling or vice versa in Custodiol cardioplegia and both processes may have created a situation that consumes ATP and/or block ATP synthesis. In other words, as Custodiol reduced the ATP level in the tissue, the inflammation also increased and associated tissue remodelling continued.
Protein-bound sialic acid is known to control many cellular processes. Hence, the removal of sialic acid from the cellular surface may cause a result that affects cellular functions [Citation28]. There was no significant difference in sialic acid level in the findings of our study. Hence, it is difficult to comment against or in favour of any solution. However, all solutions may have the same effect on glycoproteins.
In a transplantation model, an imbalance between antioxidants and oxidants following Custodiol administration has shown to be associated with oxidative stress [Citation29]. Lange et al. similarly showed the relationship between oxidative stress and inflammation during Custodiol-induced ischemia [Citation30]. Manju and Nair showed that ROS formation in the left ventricular papillary muscle weakened the contractile force and this increase was associated with a decrease in ATP production [Citation31]. Therefore, in our study, the weakening effect of Custodiol in contractions may be mediated by the release of released ROS, and this process is explained as the suppression of oxidative stress-related ATP production.
In the study of del Nido and St. Thomas cardioplegia in adult surgery, it was reported that del Nido provides shorter cross-clamp and cardiopulmonary bypass time, and no additional dose cardioplegia was required. Furthermore, del Nido cardioplegia solution was considered to protect the left ventricular ejection fraction [Citation32]. In our experimental study, no significant difference was observed in cellular and functional levels between del Nido and St. Thomas cardioplegia solution administration. This points out that any solutions may be preferred unless there is a special case.
Despite the negative studies on it as mentioned above, there are studies about better preservative properties of Custodiol in the literature. In the study of Lee et al. Celsior and histidine-tryptophan-ketoglutarate cardioplegia solutions were compared according to their effects on elderly and young donor hearts [Citation33]. High levels of tissue ATP were found in the elderly donor group in the application of Custodiol cardioplegia. Therefore, Custodiol cardioplegia may be appropriate in elderly people.
Conclusion
Cardioplegic solutions should be evaluated not only in terms of providing effective surgery but also in terms of providing effective protection against cellular damage processes. As a result of our study, we proved that Custodiol used as crystalloid cardioplegic solutions had the weakest protection in inflammation, oxidative stress and myocardial contractile strength compared to St. Thomas and del Nido cardioplegia. Comprehensive studies are needed to determine the processes that may occur due to damage in the organ to be transplanted and to change the contents of the cardioplegia when necessary.
Acknowledgement
del Nido cardioplegia solution was gifted by Dr. Utku Unal to use in the study.
Disclosure statement
None of the authors have any conflicts of interest to disclose.
Additional information
Funding
References
- Kamoda Y, Fujino Y, Tanioka Y, et al. Ischemically damaged heart after preservation by the cavitary two-layer method as a possible donor in rat heart transplantation. J Heart Lung Transplant. 2007;26(12):1320–1325.
- Dhital KK, Chew HC, Macdonald PS. Donation after circulatory death heart transplantation. Curr Opin Organ Transplant. 2017;22(3):189–197.
- Caus T, Izquierdo M, Lan C, et al. Simultaneous study of metabolism and function following cardioplegic arrest: a novel method of evaluation of the transplanted heart in the rat. J Heart Lung Transplant. 2001;20(5):575–582.
- Guibert EE, Petrenko AY, Balaban CL, et al. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother. 2011;38(2):125–142.
- Siddiqi S, Blackstone EH, Bakaeen FG. Bretschneider and del Nido solutions: are they safe for coronary artery bypass grafting? If so, how should we use them? J Card Surg. 2018;33(5):229–234.
- Hoenicke EM, Peterseim DS, Ducko CT, et al. Donor heart preservation with the potassium channel opener pinacidil: comparison with University of Wisconsin and St. Thomas’ solution. J Heart Lung Transplant. 2000;19(3):286–297.
- Parolari A, Rubini P, Cannata A, et al. Endothelial damage during myocardial preservation and storage. Ann Thorac Surg. 2002;73(2):682–690.
- Minasian SM, Galagudza MM, Dmitriev YV, et al. Preservation of the donor heart: from basic science to clinical studies. Interact CardioVasc Thorac Surg. 2015;20(4):510–519.
- Soler F, Fernández-Belda F, Pérez-Schindler J, et al. PDE2 activity differs in right and left rat ventricular myocardium and differentially regulates β2 adrenoceptor-mediated effects. Exp Biol Med. 2015;240(9):1205–1213.
- Sydow G. A simplified quick method for determination of sialic acid in serum. Biomed Biochim Acta. 1985;44(11–12):1721–1723.
- Bretschneider HJ. Survival time and recuperative time of the heart in normothermia and hypothermia. Verh Dtsch Ges Kreislaufforsch. 1964;30:11–34.
- Braimbridge MV, Chayen J, Bitensky L, et al. Cold cardioplegia or continuous coronary perfusion? Report on preliminary clinical experience as assessed cytochemically. J Thorac Cardiovasc Surg. 1977;74(6):900–906.
- del Nido PJ, Wilson GJ, Mickle DA, et al. The role of cardioplegic solution buffering in myocardial protection. A biochemical and histopathological assessment. J Thorac Cardiovasc Surg. 1985;89(5):689–699.
- Kim K, Ball C, Grady P, et al. Use of del Nido cardioplegia for adult cardiac surgery at the Cleveland Clinic: perfusion implications. J Extra Corpor Technol. 2014;46(4):317–323.
- Jahania MS, Sanchez JA, Narayan P, et al. Heart preservation for transplantation: principles and strategies. Ann Thorac Surg. 1999;68(5):1983–1987.
- Lebon JS, Couture P, Colizza M, et al. Myocardial protection in minimally invasive mitral valve surgery: retrograde cardioplegia alone using endovascular coronary sinus catheter compared with combined antegrade and retrograde cardioplegia. J Cardiothorac Vasc Anesth. 2019;33(5):1197–1204.
- Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109(4):315–323.
- Agnic I, Filipovic N, Vukojevic K, et al. Effects of isoflurane postconditioning on chronic phase of ischemia-reperfusion heart injury in rats. Cardiovasc Pathol. 2015;24(2):94–101.
- Bojan M. Recent achievements and future developments in neonatal cardiopulmonary bypass. Paediatr Anaesth. 2019;29(5):414–425.
- Hernandez-Cascales J. Resveratrol enhances the inotropic effect but inhibits the proarrhythmic effect of sympathomimetic agents in rat myocardium. PeerJ. 2017;5:e3113.
- Carmo HPD, Reichert K, Carvalho DD, et al. Lidocaine and pinacidil added to blood versus crystalloid cardioplegic solutions: study in isolated hearts. Braz J Cardiovasc Surg. 2018;33(3):211–216.
- Bae HK, Lee H, Kim KC, et al. The effect of sildenafil on right ventricular remodeling in a rat model of monocrotaline-induced right ventricular failure. Korean J Pediatr. 2016;59(6):262–270.
- Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol. 2012;44(3):98–103.
- Han J, Wang D, Yu B, et al. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid Med Cell Longev. 2014;2014:134862.
- Yang CM, Lee IT, Lin CC, et al. c-Src-dependent MAPKs/AP-1 activation is involved in TNF-α-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Biochem Pharmacol. 2013;85(8):1115–1123.
- Bradham WS, Bozkurt B, Gunasinghe H, et al. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53(4):822–830.
- Zheng XZ, Zhou JL, Ye J, et al. Cardioprotective effect of novel sulphonamides-1,3,5-triazine conjugates against ischaemic-reperfusion injury via selective inhibition of MMP-9. Chem Biol Drug Des. 2016;88(5):756–765.
- Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol. 2012;94(4):887–905.
- Renner A, Sagstetter MR, Götz ME, et al. Heterotopic rat heart transplantation: severe loss of glutathione in 8-hour ischemic hearts. J Heart Lung Transplant. 2004;23(9):1093–1102.
- Lange V, Renner A, Sagstetter MR, et al. Heterotopic rat heart transplantation (Lewis to F344): early ICAM-1 expression after 8 hours of cold ischemia. J Heart Lung Transplant. 2008;27(9):1031–1035.
- Manju L, Nair RR. Magnesium deficiency augments myocardial response to reactive oxygen species. Can J Physiol Pharmacol. 2006;84(6):617–624.
- Mishra P, Jadhav RB, Mohapatra CK, et al. Comparison of del Nido cardioplegia and St. Thomas Hospital solution - two types of cardioplegia in adult cardiac surgery. Polish J Cardio-Thorac Surg. 2016;4(4):295–299.
- Lee S, Huang CS, Kawamura T, et al. Histidine-tryptophan-ketoglutarate or celsior: which is more suitable for cold preservation for cardiac grafts from older donors? Ann Thorac Surg. 2011;91(3):755–763.