Abstract
Background
Systemic pro-coagulatory and pro-inflammatory factors are critical factors in acute pulmonary embolism (APE). Recently the systemic immune-inflammation index (SII) has emerged as a new inflammatory and prognostic marker. We aimed to determine whether there is a relationship between SII and the severity of the APE. Methods. A total of 442 patients with APE, 202 women (45.7%) with an average age of 64 ± 16, were included in this retrospective observational study. APE severity was classified as massive (high risk), submassive (intermediate risk), and nonmassive (low risk). On admission, blood samples were collected for SII and other laboratory measurements. The SII was defined as platelet × neutrophil/lymphocyte counts. Results. SII levels were higher in patients with massive APE and gradually increased from nonmassive to massive APE (p < .001). SII was also significantly higher in patients with in-hospital death. Multivariable analysis showed that SII was an independent predictor for massive APE (Odds ratio 1.005 (95% CI 1.002–1.007), p < .001), together with C-reactive protein and cardiac troponin. In the receiver operating characteristic curve, the optimal cutoff value of SII to predict a massive APE was 1161, with 91% sensitivity and 90% specificity (area under the curve: 0.957). Conclusion. Our findings support an association between a higher SII level and a massive APE. As a simple biomarker, SII is an independent predictor of more severe disease in patients with APE. SII is a more powerful tool than traditional inflammatory markers for predicting the severity of disease in these patients
Introduction
Acute pulmonary embolism (APE) is one of the leading causes of cardiovascular mortality [Citation1]. With a different range of clinical presentations, definitive diagnosis and timely risk stratification are very important. Patients are commonly risk-stratified based on blood pressure, the Pulmonary Embolism Severity Index (PESI), echocardiographic evidence of right ventricular (RV) overload, lower extremity venous Doppler ultrasonography, computed tomography pulmonary angiography, and biomarker evidence of RV ischemia [Citation2,Citation3]. These markers, however, are cumbersome or costly [Citation4]. Several studies confirmed the inflammatory response in APE suggesting the potential value of inflammatory markers in the diagnosis and prognosis in such cases [Citation5–8]. These inflammatory response tools are readily available from routine laboratory studies and provide important information about systemic inflammation status.
The levels of pro-coagulatory and pro-inflammatory factors originating from platelets and leukocytes are increased in the setting of APE [Citation9]. This acute inflammatory response leads to an increase in platelet activation and neutrophil recruitment and has been associated with poor prognosis and short-term mortality in patients presenting with APE [Citation10]. On the other hand, lymphocyte count decreases in response to adrenaline and glucocorticoids released during a sympathetic response during the course of the disease [Citation11]. With the increasing understanding of APE-related inflammation, systemic inflammation-based indexes, such as neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein level, have been clearly demonstrated to predict the prognoses of APE patients [Citation5–8]. The systemic immune-inflammation index (SII), which is a parameter integrates neutrophils, platelets and lymphocytes, has recently been assessed to predict the clinical outcomes in various cancers [Citation12–14]. However, the relationship between SII and clinical outcomes in patients with APE remains unclear. The aim of this study was to investigate the utility of SII to predict the severity of disease in patients with PE.
Materials and methods
Study population
The study population comprised consecutive 454 patients with APE admitted between 2015 and 2019. The diagnosis of APE was confirmed by computed tomography pulmonary angiogram. APE was diagnosed when symptoms had been present for no longer than 14 days before the diagnosis. Patients’ demographics, comorbidities, presenting symptoms, medications, interventions, and outcomes were collected. In all cases, venous peripheral blood samples for measurement of SII and other routine laboratory parameters were drawn on admission before the treatment. Transthoracic echocardiography was also performed on admission. Patients were excluded from further analysis if they had an active condition at the time of APE diagnosis that could significantly influence blood cell count, including sepsis, active infection, chronic inflammatory conditions, malignancy, and use of immunosuppressive therapy. After excluding 12 patients, a total of 442 patients were included in the final analysis.
Definitions
APE severity was classified as massive (high risk), submassive (intermediate risk), and nonmassive (low risk) according to European Society of Cardiology Guidelines [Citation15,Citation16], based on systemic systolic blood pressure on admission and the presence of RV dysfunction at echocardiography and elevated plasma troponin levels. Massive APE was characterized by systemic hypotension (defined as a systolic arterial pressure <90 mm Hg or a drop in systolic arterial pressure of at least 40 mm Hg for at least 15 min which is not caused by new-onset arrhythmias) or shock (manifested by evidence of tissue hypoperfusion and hypoxia, including an altered level of consciousness, oliguria, or cool, clammy extremities) [Citation2,Citation3]. Patients who are hemodynamically stable but with RV dysfunction or hypokinesis confirmed by echocardiography were classified as submassive APE. All other cases were included with the nonmassive APE group.
All patients received standard anticoagulant therapy with intravenous unfractionated heparin or subcutaneous body weight-adjusted dose of low molecular weight heparin. High-risk APE was an indication for thrombolysis (infusion of recombinant tissue plasminogen activator). Hemodynamic deterioration in initially stable patients was also considered an indication for thrombolysis. The SII was defined as platelet × neutrophil/lymphocyte counts [Citation12]. The Simplified PESI (sPESI) score was calculated based on age >80 years, history of cancer, history of chronic cardiopulmonary disease, heart rate 110/minute, systolic blood pressure <100 mm Hg, and arterial oxygen saturation <90% [Citation17]. RV overload was diagnosed when echocardiography showed RV/left ventricle >0.6 with RV free wall hypokinesis, and/or an elevated tricuspid valve pressure gradient >30 mm Hg with a shortened acceleration time of pulmonary ejection <80 ms. The protocol of this study was approved by the Local Ethical Committee. Informed consent for patient information to be used in this study was not obtained because the institutional review board provided a waiver of informed consent to conduct this retrospective research.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Company, Chicago, IL) for Windows. The Shapiro–Wilk test was used to assess the normality of distribution of continuous variables. Student or Mann–Whitney U tests were used for comparisons between two groups, while comparisons between more than two groups were performed by analysis of variance (ANOVA) or Kruskal–Wallis tests. Data characterized by a normal distribution are expressed as mean ± SD. Because some of the variables were not normally distributed, the Mann–Whitney U test was used for comparison of continuous variables which were listed as median and interquartile range (IQR). The Chi-square test or Fisher’s exact tests were used to compare both groups on categorical variables, which were then summarized using counts and percentages. Spearman’s correlation was performed to analyze the correlations between SII and other parameters. The optimal cutoff value of SII to predict a massive APE was assessed by calculating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of the occurrence of massive APE. The goodness-of-fit of the model was examined by the Hosmer–Lemeshow test. A p-value <.05 was considered statistically significant.
Results
Among the total of 442 APE patients (average age of 64 ± 16), 240 (45.7%) were women. A comparison of clinical and laboratory parameters according to the APE subgroups is summarized in . Upon classification of the presenting symptoms of the patients as dyspnea, hemoptysis, and chest pain, it was observed that the presenting complaint of dyspnea was significantly higher in the massive APE group compared to the other groups. However, there were no statistically significant differences between the groups in terms of the number of patients presenting with hemoptysis and chest pain. A history of cardiac diseases (coronary artery disease, heart failure) was also detected at a higher rate in the massive PTE group. Patients with massive APE were older and they had a higher frequency of diabetes mellitus and a higher WBC count, neutrophil count, platelet count, cardiac troponin, SII, D-dimer, NT-proBNP levels, PAPs, Wells score, a higher frequency of in-hospital mortality but lower lymphocyte count, systolic blood pressure, and diastolic blood pressure. In , the comparison of in-hospital mortality with clinical and laboratory variables are presented. As expected, the frequency of in-hospital mortality was higher in older and diabetic patients. The level of CRP, NT-proBNP, troponin, SII, andsPESIweresignificantlyhigher in-hospital mortality group. Then, we investigated the predictors of massive APE, using univariate and multivariate analyses (). In the multivariate logistic regression model (n = 442, p from Hosmer and Lemeshow = .751), we found that SIIon admission, together with troponin (OR 1.012, p = .029) and CRP (OR 1.034, p = .038), was independently associated with a massive APE (OR 1.005, 95% CI 1.003–1.007, p < .001) after adjusting for all confounding factors (patients’ age, troponin level, white blood cell count, CRP, NT-proBNP, D-dimer, SII, Wells score, and the presence of diabetes mellitus).
Table 1. The association of the variables with the acute pulmonary embolism severity.
Table 2. The relationship between in-hospital mortality and clinical and laboratory variables.
Table 3. Logistic regression analyses showing independent predictors of massive acute pulmonary embolism.
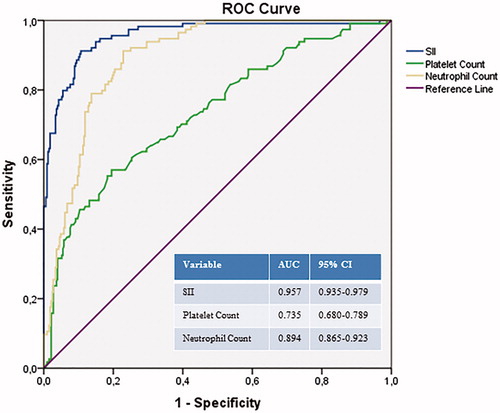
ROC curve analyses of SII, neutrophil count, and platelet count in predicting massive APE were performed. According to the ROC curve, the ideal SII cutoff value was >1161, with 91% sensitivity and 90% specificity. The areas under the ROC curve for SII, neutrophil count, and platelet count were 0.957 (95% CI for the area between 0.935 and 0.979, p < .001), 0.894 (95% CI for the area between 0.865 and 0.923, p < .001), and 0.735 (95% CI for the area between 0.680 and 0.789, p < .001), respectively (). Pearson correlation analysis showed a significant positive correlation between SII and troponin (r = .172, p = .005), and CRP (r = .231, p < .001).
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between SII level and severity of APE. We found that SII was identified as a strong independent predictor of massive APE, with an optimal cut-off value of >1161. We also found an association between the severity of disease and CRP and Troponin levels. Thus, SII may be a useful, novel biomarker in predicting the severity of APE in addition to older inflammatory and prognostic biomarkers such as CRP and troponin.
Deep venous thrombosis and APE are considered as two different clinical manifestations of a single disease. Approximately 90% of symptomatic APEs are reported to originate from thrombus located in the venous system of the lower extremity [Citation18,Citation19]. To the contrary of the previous belief that venous thromboembolism is pathophysiologically distinct from atherothrombotic disorders, it is now suggested that it should be considered a part of a “pan-cardiovascular syndrome” [Citation20] that includes coronary artery disease, peripheral arterial disease, and cerebrovascular disease [Citation21], as they all share the same underlying hypercoagulability, endothelial injury as well as inflammation [Citation22]. Serum levels of pro-coagulatory and pro-inflammatory microparticles originating from platelets, leukocytes, and endothelial cells are increased in the setting of APE [Citation22]. This acute inflammatory response leads to an increase in further platelet activation and neutrophil recruitment and has been associated with poor prognosis and short-term mortality in patients presenting with PE [Citation10]. In addition, lymphocyte count decreases in response to adrenaline and glucocorticoids released during a sympathetic response [Citation11]. Araz et al. investigated the predictive value of serum high sensitivity-CRP levels for outcomes of APE, and they showed that high serum levels of high sensitivity-CRP had a markedly high relation with mortality [Citation6]. In a previous study, CRP was associated with RV dysfunction, which is a predictor of prognosis in APE and it was suggested as a promising biomarker for risk stratification of APE [Citation5]. In accordance with the results of that study, in our study, the CRP level was also independently associated with massive APE. A recent study evaluated the association between a crude marker of inflammation, serum albumin and massive versus non-massive APE, and showed that albumin was associated with massive APE [Citation7]. The researchers suggested that this relationship is likely a proxy for a higher inflammatory state in massive compared with non-massive APE. In another recent study, Phan et al. investigated the utility of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) to predict all-cause mortality in patients presenting with APE [Citation8]. They found that elevated NLR and PLR were associated with all-cause mortality in APE. However, these inflammation biomarkers only integrate two inflammatory cells. The SII, which is an accurate systemic thrombo-inflammatory index based on platelet, neutrophil, and lymphocyte counts, is a novel indicator that can predict the clinical outcomes of cancer patients [Citation12–14]. We thought that this index may reflect the degree of systemic inflammation and thrombogenicity more accurately. This is the first study to link SII with the severity of APE. In the present study, we found that SII was independently correlated with massive APE, and hence it serves as a prognostic marker. Although elevated SII levels robustly associate with the severity of APE, this may not necessarily be a causal association. It seems more likely that the inflammatory state seen in venous thromboembolism is a consequence of an underlying causal pathway, also leading to a hypercoagulant state and eventually to APE [Citation23].
In a previous study, CRP was associated with RV dysfunction, which is a predictor of prognosis in APE and it was suggested as a promising biomarker for risk stratification of APE [Citation5]. In accordance with the results of that study, in our study, CRP level was also independently associated with massive APE.
Elevation of troponin and its prognostic significance in APE was reported previously in many studies [Citation24–26]. In accordance with current literature data, in our study patients with massive APE significantly elevated troponin concentrations were detected and troponin was independently associated with massive APE. It seems justified to suggest that hemodynamic factors such as increased RV overload, hypotension, and hypoxemiacan contribute to myocardial injury.
Study limitations
There were some limitations in our study. Firstly, this research was a retrospective study. Secondly, SII was measured at hospital admission after the diagnosis of APE so it is hard to differentiate whether increased SII level is a cause or a consequence of the thromboembolic process. It would be great if we could evaluate thrombophilic gene polymorphisms such as Factor V LEIDEN, methylenetetrahydrofolate reductase (MTHFR) in our study population. But we do not have this data of patients. Further studies in which thrombophilic gene polymorphisms are also evaluated in this patient population are required.
Conclusion
Our study suggests that SII, which can be easily calculated from a complete blood count test, is an independent predictor for massive APE, and given its very high sensitivity and specificity, therefore its reliability, the SII is superior to other inflammation-based indexes. However, prospective multicenter clinical trials are required to validate the findings in our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711–1717.
- Kucher N, Goldhaber SZ. Risk stratification of acute pulmonary embolism. Semin Thromb Hemost. 2006;32(8):838–847.
- Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J. 2000;21(16):1301–1336.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J RespirCrit Care Med. 2014;189(6):718–726.
- Abul Y, Karakurt S, Ozben B, et al. C-reactive protein in acute pulmonary embolism. J Investig Med. 2011;59(1):8–14.
- Araz O, Yilmazel Ucar E, Yalcin A, et al. Predictive value of serum Hs-CRP levels for outcomes of pulmonary embolism. Clin Respir J. 2016;10(2):163–167.
- Omar HR, Mirsaeidi M, Rashad R, et al. Association of serum albumin and severity of pulmonary embolism. Medicina. 2020;56(1):26.
- Phan T, Brailovsky Y, Fareed J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict all-cause mortality in acute pulmonary embolism. Clin Appl Thromb Hemost. 2020;26:107602961990054.
- Chung T, Connor D, Joseph J, et al. Platelet activation in acute pulmonary embolism. J Thromb Haemost. 2007;5(5):918–924.
- Jo JY, Lee MY, Lee JW, et al. Leukocytes and systemic inflammatory response syndrome as prognostic factors in pulmonary embolism patients. BMC Pulm Med. 2013;13(1):74.
- Ince LM, Weber J, Scheiermann C. Control of leukocyte trafficking by stress-associated hormones. Front Immunol. 2018;9:3143.
- Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272.
- Diao P, Wu Y, Li J, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. J Transl Med. 2018;16(1):365.
- Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. 2020;24(3):610–618.
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276–2315.
- Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18(2):129–138.
- Cooper TJ, Prothero DL, Gillett MG, et al. Laboratory investigation in the diagnosis of pulmonary thromboembolism. Q J Med. 1992;83(301):369–379.
- Perrier A, Bounameaux H. Cost-effective diagnosis of deep vein thrombosis and pulmonary embolism. Thromb Haemost. 2001;86(07):475–487.
- Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23):I22–I30.
- Piazza G, Ridker PM. Is venous thromboembolism a chronic inflammatory disease? Clin Chem. 2015;61(2):313–316.
- Piazza G, Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121(19):2146–2150.
- Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–285.
- Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30(8):1672–1678.
- Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116(4):427–433.
- Kostrubiec M, Pruszczyk P, Bochowicz A, et al. Biomarker-based risk assessment model in acute pulmonary embolism. Eur Heart J. 2005;26(20):2166–2172.
- Ng AC, Yong AS, Chow V, et al. Cardiac troponin-T and the prediction of acute and long-term mortality after acute pulmonary embolism. Int J Cardiol. 2013;165(1):126–133.