Abstract
Objective
Patients undergoing carotid endarterectomy (CEA) may experiment neurologic deficits during the carotid cross-clamping due to secondary cerebral hypoperfusion. An associated risk of postoperative stroke incidence is also well established. This work aimed to assess the postoperative adverse events related to neurologic deficits in the awake test after clamping and to determine its predictive factors. Methods. From January 2012 to January 2018, 79 patients from a referral hospital that underwent CEA with regional anesthesia for carotid stenosis and manifested neurologic deficits were gathered. Consecutively selected controls (n = 85) were submitted to the same procedure without developing neurological changes. Postoperative complications such as stroke, myocardial infarction, all-cause death, and Clavien–Dindo classification were assessed 30 days after the procedure. Univariate and binary logistic regressions were performed for data assessment. Results. Patients with clamping associated neurologic deficits were significantly more obese than the control group (aOR = 9.30; 95% CI: 2.57–33.69; p = .01). Lower degree of ipsilateral stenosis and higher degree of contralateral stenosis were independently related to clamping intolerance (aOR = 0.70; 95% CI: 0.49–0.99; p = .047 and aOR = 1.30; 95% CI: 1.06–1.50; p = .009, respectively). Neurologic deficits were a main 30-day stroke predictor (aOR = 4.30; 95% CI: 1.10–16.71; p = .035). Conclusions. Neurologic deficits during carotid clamping are a predictor of perioperative stroke. Body mass index > 30 kg/m2, a lower degree of ipsilateral stenosis, and a higher degree of contralateral stenosis are independent predictors of neurologic deficits and, therefore, might play a role in the prevention of procedure-related stroke.
Introduction
Carotid endarterectomy (CEA) is the recommended treatment for symptomatic carotid stenosis [Citation1]. The procedure implies carotid cross-clamping (CACC), which might lead to the intraoperative manifestation of neurologic deficits (IND) due to the hypoperfusion caused by the former and also exposes the patient to further embolism [Citation2]. Prolonged CACC time was demonstrated to augment the risk of 30-day stroke and death, with an OR 1.1 for each 10-minute increase [Citation3]. It may also affect hemodynamic stability, and both hypertension and hypotension were associated with stroke postoperatively [Citation4]. CACC reduces blood flow to the brain leading to hypoperfusion, which can be aggravated if collateral circulation is compromised [Citation2,Citation5–7]. Preoperative dual anti-aggregation has significantly reduced the incidence of postoperative embolic stroke [Citation2]. Regarding hemodynamic stroke, recommendations have been made to avoid hypotension and adjust blood pressure into high mean arterial pressure values during CEA [Citation4,Citation8].
CEA can be performed under general or regional anesthesia (RA), without significant differences regarding mortality or stroke rates [Citation9]. However, RA is associated with less resort to shunt [Citation10,Citation11], shorter surgical time [Citation12], and lower risk of coronary events [Citation3]. A neurological examination is the most accurate strategy for cerebral monitoring during CEA [Citation10,Citation13,Citation14]. Post-clamping IND are associated with higher ischemic event rates [Citation6,Citation15]. Resort to shunting during CEA has been recommended after the occurrence of IND associated to CACC [Citation16]. Described predictive factors for shunting include female gender and age superior to 75 years [Citation17], contralateral carotid occlusion [Citation7,Citation17,Citation18] and moderate ipsilateral internal carotid artery stenosis [Citation7,Citation18], symptomatic presentation [Citation7], arterial hypertension [Citation17] and need for shunting in first procedure [Citation19]. Contralateral common artery blood flow >619 mL/min and history of contralateral carotid surgery were reported as protective for cerebral ischemia [Citation17].
Cerebral perfusion is preserved during RA surgery by a physiological rise in systemic blood pressure and cerebral oxygenation after the carotid clamping. However, during general anesthesia, autoregulation is compromised in some patients. Therefore, the preservation of cerebral autoregulation might contribute to the further reduction of ischemic episodes [Citation20].
Most of the current literature focuses on IND in the setting of general anesthesia. Still, the neurologic examination of the awake patient is considered the best reliable method to assess IND. This study sought to evaluate reasonable predictors of perioperative neurologic implications of CACC during CEA performed under RA. Additionally, potential adverse events associated with IND were also assessed.
Materials and methods
Study population
From January 2012 to January 2018, all patients from a tertiary referral center who underwent CEA under RA for carotid stenosis were reviewed. Analysis of all patients (n = 79) who presented alterations in the neurologic examination after internal carotid artery clamping during CEA was performed. Control patients, who were submitted to the same procedure without presenting neurologic alterations, were consecutively selected. Additionally, the center exclusion criteria for RA were synchronous cardiac surgery and patients a priori unwilling to stay awake during the procedure, resulting in the use of RA in 97% of cases.
Demographics and comorbidities of the selected patients along with 30-day post procedure adverse events were recorded. The study protocol was approved by the local Ethics Committee (Protocol 248-18) and respected the Declaration of Helsinki. Patient informed consent was waived by the same committee. The study’s database is registered and available on https://www.researchregistry.com. Unique identifying number – Research Registry4929.
Perioperative setting
All patients were subjected to preoperative Doppler ultrasound (US) or angio-CT to define the topography and severity according to velocimetric criteria (50–70%; 70–90%; 90–99%), as well as to evaluate the other vessels involved in cerebral vascularization [Citation21]. Patients were evaluated by a vascular surgeon and an anesthesiologist before the surgery, and were under single antiplatelet therapy (>95% acetylsalicylic acid 100 mg) and a statin for at least two days before surgery. Anticoagulation was managed according to present clinical guidelines [Citation22]. None of the patients were submitted to thrombolysis or mechanical thrombectomy prior to surgery.
RA by cervical block was performed with the patient in the supine position, and head turned opposingly to the surgery side. A 22-gauge insulated needle was perpendicularly inserted under US guidance in most cases. After that, 4–5 mL of ropivacaine 0.5% was administered per spinal level (C2–C4) in a total of 12–15 mL (deep cervical blockade) and/or 5 mL of ropivacaine 0.5% at the posterior border of the midportion of the sternocleidomastoid muscle (superficial cervical blockade) were injected [Citation23].
Intraoperative monitoring consisted of consecutive brief awake neurological examinations and continuous anesthetic surveillance during the procedure and arterial clamping, respectively [Citation11]. Cerebral oximeter (INVOSTM) was additionally placed in all cases after 2013, nonetheless, neurological examination always prevailed in the decision [Citation24,Citation25]. According to these criteria, the rate of IND during CEA in this center is 8%/year.
Mean arterial pressure was routinely measured using a 20-gauge catheter placed in the radial artery. Until the carotid artery was declamped, mean arterial pressure was stabilized between baseline values to 20% above. If required, vasoactive medication was administered: ephedrine 5–10 mg bolus for hypotension and atropine 1 mg bolus for bradycardia. All patients underwent surgery with 2 L/min of oxygen by nasal cannula, and a peripheral HbO2 saturation of at least 95% was aimed. Concerning patients who developed critical hypotension, unworkable agitation, or neurologic deficits were converted to general anesthesia with propofol.
Surgical technique and postoperative surveillance
Surgery consisted of CEA followed by patch angioplasty, direct suture or eversion. Shunt technique was applied based on the surgeons’ experience. The Javid shunt was used in the period 2012–2015 and changed to the Pruitt-Inahara® 2015–2018 due to logistic options [Citation26]. Findings regarding the selective or non-use of shunt are described elsewhere [Citation26]. Once the surgery was performed, patients were subjected to continuous monitoring for 24 h in a post-anesthetic care unit. A brain CT was then performed if any neurologic alteration was diagnosed.
The result of the surgery was assessed in the subsequent 30–90 days by clinical examination and Doppler US. Additionally, the vascular surgery team has a stroke rate in CEA of 1.8% and a stroke/mortality rate of 1.8% in symptomatic patients [Citation27,Citation28].
Statistical analysis
The required sample for a two-sided test for non-superiority was calculated resorting to WinPepi® V11.65 [Citation29], aiming for statistical power (β) of 90% and an α < 0.05. The described neurologic event rate in shunted patients/cerebral malperfusion patients is 4%, and an event rate of 1.8% for the controls was assumed [Citation16,Citation28,Citation30]. A prevalence of 50% of INDs in the sample with a ratio of case to controls of 1.1:1 was considered. An event rate difference of 20% between groups was established, resulting in an estimated minimum sample of 72 patients.
Statistics were performed with SPSS 25.0 (IBM Corp., release 2017; IBM SPSS Statistics for Windows, version 25.0, Armonk, NY). Continuous and categorical data were subject to univariate analysis through Student’s t-test, χ2 or Fisher’s test, respectively. Mann–Whitney’s U test was used to assess ordinal skewed variables and was presented as median and 5–95% confidence intervals (CIs). Categorical and continuous variables are presented as percentages and as mean ± standard deviation, respectively. The significance level was set to p value <.05 and adjusted odds ratio (aOR) together with 95% CI were calculated.
Multivariable analysis was performed using binary logistic regression by the dimension reduction method. Variables with clinical relevance included in the multivariable analysis were those associated with the group with post-clamping neurologic changes in univariate analysis (variables with p<.15 were included).
Definitions
Symptomatic carotid stenosis was defined according to the clinical practice guidelines of the European Society for Vascular Surgery [Citation31]. Post-clamping deficits were defined as any persistent alteration at neurologic examination during the CACC and resistant to hemodynamic adjustment [Citation4]. All the postoperative neurologic events were confirmed and evaluated by an experienced neurologist.
Postprocedural stroke was defined as an episode of acute neurological dysfunction presumed to be caused by ischemia or hemorrhage, persisting at least 24 h or until death [Citation32]. Surgical hematoma was defined as a significant cervical blood collection associated to respiratory airway compression with the need for surgical intervention.
Clavien–Dindo classification was used for grading adverse events that occur as a result of surgical procedures, and its main characteristic is that the severity of a complication is graded based on the type of therapy required to treat it [Citation33]. In the present study, adverse events were composed of the 30-day rate of stroke, hyperperfusion syndrome, postprocedure hypotension with the need for adrenergic support, and surgical hematoma. No 30-day death or myocardial infarction events were recorded in this cohort.
Posterior circulation disease was defined as the presence of vertebral artery stenosis with hemodynamic significance on US or superior to 50% in the angio-CT in either side of vertebrobasilar circulation [Citation34].
Results
Demographic and clinical data
The study population included 131 men (80%) and 33 women (20%) with a mean age of 69 ± 9.30 years (range 45–89). No significant differences between the post-clamping deficit group and the controls regarding gender was found (24% vs. 16%, p = .226). Mean age of cases was significantly higher than in the control group (71 ± 9.30 years vs. 68 ± 9.0 years, p = .042), although not confirmed by multivariable analysis. Of the total sample, 34% of patients aged >75 years, of which 61% presented clamping associated IND (p = .021) ().
Table 1. Population demographics and comorbidities.
Cardiovascular risk-factors were discriminated for both groups, demonstrating homogeneity across proportions except for body mass index > 30 kg/m2, which was significantly more frequent in the group with IND (24% vs. 5%; p = .0001) (). Concerning the use of anti-hypertensive agents as chronic therapy, only the use of calcium channel blockers reported significant association with IND (p = .019).
American Society of Anesthesia physical status classification (ASA) demonstrated higher grades for the group with IND (2.96 ± 0.406 vs. 2.79 ± 0.514, p = .017). Both groups were comparable in terms of preoperatory hemoglobin (g/dL) (p = .219) ().
Neurovascular symptoms and intraoperative features
Regarding the onset of IND, 68 patients (86%) manifested symptoms in the first 5 min after CACC, with a second late incidence peak at 10 min. Concerning the CS, 43% of the patients were symptomatic (33% stroke and 10% transient ischemic attack, with no difference between groups, p = .488). The median time between the event and the surgery was 17.5 days (interquartile range (IQR) 10.25–57.75) for the control group and 13.5 days (IQR 10–39.25) for the group with IND (p = .795) (). Similarly, no significant results were found concerning 30-day stroke (p = .152) or postoperative complications (p = .152).
Table 2. Intraoperative and neurovascular predictors.
Regarding the degree of stenosis, ipsilateral stenosis was significantly less marked in the IND group (82.5%±10.78% vs. 86.0%±9.54%, p = .031). On the other side, the degree of contralateral stenosis was significantly superior in patients who presented clamping associated IND (68.9%±22.90% vs. 61.7%±17.02%, p = .026). Both groups were comparable in terms of surgery time (110 ± 38.00 vs. 117 ± 55.20 min, p = .408), but significantly differed for clamping time (48 ± 23.106 vs. 37 ± 21.508, p = .007) ().
Multivariable analysis and confounding
After multivariable analysis, the variables maintaining association with IND were BMI > 30 kg/m2 (aOR = 9.30; 95% CI: 2.57–33.69; p = .01), lower ipsilateral stenosis (aOR = 0.70; 95% CI: 0.49–0.99; p = .047) and higher contralateral stenosis (aOR = 1.30; 95% CI: 1.06–1.50; p = .009) (R2 = 0.21; and likelihood: 186.4). Clavien–Dindo classification ≥ 2 and 30-day stroke were confirmed associations with IND as above-mentioned. Furthermore, significant results on univariate analysis for the variables age, age > 75, ASA, calcium channel blockers, conversion to general anesthesia and cranial nerve injury (CNI) were not confirmed after adjustment for confounding.
Post-clamping deficits outcomes
A significant relation between post-clamping deficits and 30-day stroke was found. Of the patients with 30-day stroke event, all with ischemic etiology, 79% manifested neurologic changes during CACC, resulting in a superior 30-day stroke rate in this group (14% vs. 3.1%, p = .017). IND was confirmed as a major stroke predictor by multivariable analysis (aOR = 4.30; 95% CI: 1.10–16.71; p = .035). Likewise, concerning patients classified with a resulting Clavien–Dindo ≥ 2, a higher incidence was also found in the group with post-clamping deficits (25% vs. 11%, p = .014), posteriorly confirmed when adjusted for confounding (aOR = 2.86; 95% CI: 1.215–6.75; p = .016). For CNI, IND did not show any significant association (9% vs. 12, p = .542) () ().
Figure 1. 30 day postoperative adverse events. ND: alterations on the neurologic examination during carotid clamping. *Confirmed by multivariable analysis.
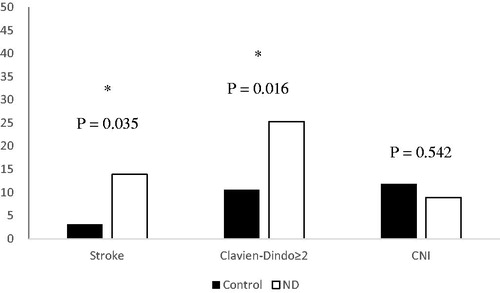
Table 3. 30-Day postoperative adverse events following carotid intervention.
Shunt was performed in 25 patients (36%) with IND and selective-shunting did not reach significative difference regarding above-mentioned outcomes [Citation26].
All cases of conversion to general anesthesia were due to IND (IND 10 (12.6%) vs. control 0, p = .001). Among IND patients, conversion to general anesthesia was not associated to 30-day stroke (p = .579).
Discussion
The reasoning for the present study is the assumption that patients developing IND during CACC are manifesting some degree of cerebral ischemia, thus having a higher risk of developing a perioperative stroke. In fact, in the present cohort, a fourfold increased risk of developing 30-day stroke in patients with IND was identified. In a study with 385 patients, Piffaretti et al. found a significant association between IND and subsequent postoperative ischemic events, denoting an aOR of 6.60 (95% CI: 1.65–26.36), p = .008 [Citation35]. Some ischemic lesions are associated with injury to the ischemic penumbra surrounding recent infarction, in addition to the traditional association between procedure-related embolization and intra- and postoperative stroke [Citation2]. The surgery itself might predispose to remaining flow turbulence and impairment of carotid baroreflex, which has been previously associated with further embolism and stroke in the short-term [Citation36,Citation37].
In the present study, inferior degrees of ipsilateral stenosis were associated with higher rates of post-clamping deficits. It is important to highlight that a stenosis greater than 90% allows for a minimal continuous brain blood flow and consequently decreases the susceptibility to low flow periods [Citation38], allowing better CACC tolerance. In stroke patients with unilateral carotid stenosis, contralateral carotid flow displays a crucial role in compensating cerebral hypoperfusion, whereas posterior circulation becomes fundamental when stenosis is bilateral [Citation17,Citation39]. In fact, patients with severe stenosis have reduced risk of transient ischemic attack/stroke, due to the presence of collateral pathways [Citation38]. A positive association was found between contralateral internal carotid stenosis and increased incidence of IND. A similar trend was found in the General Anesthesia vs. Local Anesthesia for Carotid Surgery (GALA) trial (p = .098) [Citation11], and the association of contralateral stenosis with perioperative stroke is frequent in the literature [Citation40,Citation41]. Therefore, the degree of stenosis of both sides must be taken into account during surgery planning as contralateral anatomy might influence different strategies and outcomes.
Concerning the surgery itself, CACC time was significantly lower in the group with IND. However, this finding is a consequence of neurologic deficits detection leading to quicken procedures and, therefore, it must not be interpreted as a potential risk factor. In addition to this, a report has shown clamping tolerance in patients with 40–50 min of CACC, which might explain and supports clamping times in these series [Citation42].
Age was preliminarily found to increase the risk of IND, although when adjusted for confounding, the effect was not confirmed. Actually, older patients might as well endure other comorbidities that potentially affect the results. Nonetheless, higher stroke and mortality rates among octogenarians remain acceptable and should not be labeled as “non-eligible” for surgery according to the actual evidence [Citation43].
Obese patients had a significantly ninefold higher risk of IND during CEA. It was previously reported that BMI > 30 kg/m2 is associated with increased surgical time and complications [Citation44,Citation45]. Due to the quantity of adipose tissue present in these patients, the surgical technique might display more difficulties leading to higher extensive dissection or additional manipulation and thus generating a predisposition to embolic incidents. Furthermore, these patients could present higher intolerance to the surgery or a lower threshold for dyspnea associated with a shorter neck. Kardassis et al. found an increased plaque area and thickness in common carotid artery and bulb of obese patients, suggesting higher susceptibility to inflammatory stimuli and further hemodynamic stress [Citation46], leading to an increased risk of postoperative stroke in these patients.
It was also verified that the use of calcium channel blockers was associated with an increased incidence of IND, although not confirmed by multivariable analysis. The triggered systemic vasodilation might accentuate hypotension and further cerebral hypoperfusion in addition to the CACC [Citation47]. Furthermore, these agents are associated with cerebral vasodilation after CEA, which might be detrimental for patients with reestablished/higher cerebral perfusion and higher impairment of autoregulation [Citation47]. These changes in blood flow could play a role predisposing higher stroke rates after surgery.
ASA was not comparable between controls and the group with clamping related IND, whereas it has shown significantly higher levels in the latter. This finding is supported by another report that suggests severe forms of atherosclerotic plaques in these patients. These plaques might explain the higher instability and, subsequently, the presence of more intraoperative deficits [Citation48].
While RA was not associated with better outcomes than general anesthesia, there are differences in hemodynamic stability and neurological monitoring ease [Citation23]. Only a few patients in this cohort were converted to general anesthesia ensuring the tolerability of the anesthetic method. Conversion to general anesthesia was always associated with IND in this cohort, although its prognostic implications cannot be definitely assured.
This series describes one of the most extensive series of CEA under RA with post-CACC associated neurologic deficits. The low rate of patients with IND (8%) made the prospective registry and case recruitment a hard task. Patient selection criteria did not change significantly during the follow-up, although due to the long time frame, some bias could be present. This study was performed in a large academic teaching institution (>100 CEAs per year), which might affect the external validity of the results to community hospitals that perform a large proportion of CEA. In addition, the comorbidities were comparable among groups, which weakens the chance of confounding factors. The option to shunt after the diagnosis of IND was left to the surgeon discretion, which might lead to some interference with the results, although the stroke rate was still elevated comparing to controls [Citation26].
Neurologic deficits associated to CACC was confirmed as a relevant risk factor for major postoperative adverse events. Such patients could benefit from postoperative intensive care surveillance and preoperative patient selection. BMI > 30 kg/m2, lower ipsilateral stenosis, and higher contralateral stenosis were significantly associated with a higher incidence of IND. Therefore, its management could play a critical role in the prevention of procedure-related stroke.
Future studies should focus on strategies to prevent or reduce IND after CACC. Further steps are necessary to develop a consistent score for patient selection or prompt prophylactic shunt placement among high-risk patients, reducing the exposure to symptomatic cerebral ischemia and the possibility of lifelong lesions.
Acknowledgements
Provenance and peer review: Not commissioned, externally peer-reviewed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
This study database is available in Clinical registry: https://www.researchregistry.com. Identifying number: researchregistry4929. Hyperlink to the registration: https://www.researchregistry.com/register-now#home/registrationdetails/5cf4721053761c000cc1c25a/
References
- Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(4):1–19.
- Sharpe RY, Dennis MJ, Nasim A, et al. Dual antiplatelet therapy prior to carotid endarterectomy reduces post-operative embolisation and thromboembolic events: post-operative transcranial Doppler monitoring is now unnecessary. Eur J Vasc Endovasc Surg. 2010;40(2):162–167.
- Knappich C, Kuehnl A, Tsantilas P, et al. Intraoperative completion studies, local anesthesia, and antiplatelet medication are associated with lower risk in carotid endarterectomy. Stroke. 2017;48(4):955–962.
- Stoneham MD, Thompson JP. Arterial pressure management and carotid endarterectomy. Br J Anaesth. 2009;102(4):442–452.
- Palombo D, Lucertini G, Mambrini S, et al. Subtle cerebral damage after shunting vs non shunting during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2007;34(5):546–551.
- Li J, Shalabi A, Ji F, et al. Monitoring cerebral ischemia during carotid endarterectomy and stenting. J Biomed Res. 2016;31:11–16.
- Ballotta E, Saladini M, Gruppo M, et al. Predictors of electroencephalographic changes needing shunting during carotid endarterectomy. Ann Vasc Surg. 2010;24(8):1045–1052.
- Lawrence PF, Alves JC, Jicha D, et al. Incidence, timing, and causes of cerebral ischemia during carotid endarterectomy with regional anesthesia. J Vasc Surg. 1998;27(2):329–334, discussion 35–37.
- Group GTC, Lewis SC, Warlow CP, et al. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet. 2008;372(9656):2132–2142.
- Stoughton J, Nath RL, Abbott WM. Comparison of simultaneous electroencephalographic and mental status monitoring during carotid endarterectomy with regional anesthesia. J Vasc Surg. 1998;28(6):1014–1021; discussion 21–23.
- Gough MJ, Bodenham A, Horrocks M, et al. GALA: an international multicentre randomised trial comparing general anaesthesia versus local anaesthesia for carotid surgery. Trials. 2008;9:28.
- Aziz F, Lehman EB, Reed AB. Increased duration of operating time for carotid endarterectomy is associated with increased mortality. Ann Vasc Surg. 2016;36:166–174.
- Moritz S, Kasprzak P, Arlt M, et al. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107(4):563–569.
- Hans SS, Jareunpoon O. Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg. 2007;45(3):511–515.
- Arnold M, Sturzenegger M, SchäFfler L, et al. Continuous intraoperative monitoring of middle cerebral artery blood flow velocities and electroencephalography during carotid endarterectomy. A comparison of the two methods to detect cerebral ischemia. Stroke. 1997;28(7):1345–1350.
- Chongruksut W, Vaniyapong T, Rerkasem K. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev. 2014;2014(6):CD000190.
- Kretz B, Abello N, Bouchot O, et al. Risk index for predicting shunt in carotid endarterectomy. Ann Vasc Surg. 2014;28(5):1204–1212.
- Tan TW, Garcia-Toca M, Marcaccio EJ Jr., et al. Predictors of shunt during carotid endarterectomy with routine electroencephalography monitoring. J Vasc Surg. 2009;49(6):1374–1378.
- Marrocco-Trischitta MM, Melissano G, Kahlberg A, et al. Increased incidence of cerebral clamping ischemia during early contralateral carotid endarterectomy. J Vasc Surg. 2006;43(6):1155–1161.
- McCleary AJ, Maritati G, Gough MJ. Carotid endarterectomy; local or general anaesthesia? Eur J Vasc Endovasc Surg. 2001;22(1):1–12.
- Oates CP, Naylor AR, Hartshorne T, et al. Joint recommendations for reporting carotid ultrasound investigations in the United Kingdom. Eur J Vasc Endovasc Surg. 2009;37(3):251–261.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol. 2014;31(10):517–573.
- Hakl M, Michalek P, Sevcik P, et al. Regional anaesthesia for carotid endarterectomy: an audit over 10 years. Br J Anaesth. 2007;99(3):415–420.
- Pennekamp CW, Bots ML, Kappelle LJ, et al. The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surg. 2009;38(5):539–545.
- Rocha-Neves JP, Pereira-Macedo J, Moreira AL, et al. Efficacy of near-infrared spectroscopy cerebral oximetry on detection of critical cerebral perfusion during carotid endarterectomy under regional anesthesia. Vasa. 2020;49(5):367–374.
- Rocha-Neves JM, Pereira-Macedo J, Dias-Neto MF, et al. Benefit of selective shunt use during carotid endarterectomy under regional anesthesia. Vascular. 2020;28(5):505–512.
- Lobo M, Mourao J, Afonso G. Carotid endarterectomy: review of 10 years of practice of general and locoregional anesthesia in a tertiary care hospital in Portugal. Rev Bras Anestesiol. 2015;65(4):249–254.
- Alves-Ferreira J, Rocha-Neves J, Dias-Neto M, et al. Poor long-term outcomes after carotid endarterectomy: a retrospective analysis of two Portuguese centers. Scand Cardiovasc J. 2019;53(5):266–273.
- Abramson JH. WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. Epidemiol Perspect Innov. 2004;1(1):6.
- Orlicky M, Vachata P, Bartos R, et al. A selective carotid artery shunting for carotid endarterectomy: prospective MR DWI monitoring of embolization in a group of 754 patients. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):89–92.
- Naylor AR, Ricco JB, de Borst GJ, et al. Editor's choice – management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(1):3–81.
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196.
- Rozeman AD, Hund H, Westein M, et al. Duplex ultrasonography for the detection of vertebral artery stenosis: a comparison with CT angiography. Brain Behav. 2017;7(8):e00750.
- Piffaretti G, Tarallo A, Franchin M, et al. Outcome analysis of carotid cross-clamp intolerance during carotid endarterectomy under locoregional anesthesia. Ann Vasc Surg. 2017;43:249–257.
- Bandyk DF, Kaebnick HW, Adams MB, et al. Turbulence occurring after carotid bifurcation endarterectomy: a harbinger of residual and recurrent carotid stenosis. J Vasc Surg. 1988;7(2):261–274.
- Sigaudo-Roussel D, Evans DH, Naylor AR, et al. Deterioration in carotid baroreflex during carotid endarterectomy. J Vasc Surg. 2002;36(4):793–798.
- Henderson RD, Eliasziw M, Fox AJ, et al. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31(1):128–132.
- Fang H, Song B, Cheng B, et al. Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol. 2016;16(1):39.
- Knappich C, Kuehnl A, Haller B, et al. Associations of perioperative variables with the 30-day risk of stroke or death in carotid endarterectomy for symptomatic carotid stenosis. Stroke. 2019;50(12):3439–3448.
- de Borst GJ, Moll FL, van de Pavoordt HD, et al. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg. 2001;21(6):484–489.
- Collice M, Arena O, Fontana RA, et al. Role of EEG monitoring and cross-clamping duration in carotid endarterectomy. J Neurosurg. 1986;65(6):815–819.
- Miller MT, Comerota AJ, Tzilinis A, et al. Carotid endarterectomy in octogenarians: does increased age indicate "high risk?". J Vasc Surg. 2005;41(2):231–237.
- Durup-Dickenson M, Nicolajsen CW, Budtz-Lilly J, et al. Body mass index and operating times in vascular procedures. EJVES Short Rep. 2017;35:19–23.
- Jackson RS, Sidawy AN, Amdur RL, et al. Obesity is an independent risk factor for death and cardiac complications after carotid endarterectomy. J Am Coll Surg. 2012;214(2):148–155.
- Kardassis D, Schonander M, Sjostrom L, et al. Carotid artery remodelling in relation to body fat distribution, inflammation and sustained weight loss in obesity. J Intern Med. 2014;275(5):534–543.
- Tejada JG, Taylor RA, Ugurel MS, et al. Safety and feasibility of intra-arterial nicardipine for the treatment of subarachnoid hemorrhage-associated vasospasm: initial clinical experience with high-dose infusions. AJNR Am J Neuroradiol. 2007;28(5):844–848.
- Eckstein HH, Ringleb P, Dorfler A, et al. The Carotid Surgery for Ischemic Stroke trial: a prospective observational study on carotid endarterectomy in the early period after ischemic stroke. J Vasc Surg. 2002;36(5):997–1004.
