Abstract
Objectives
To evaluate the diagnostic yield of the ECG criteria for ST-elevation myocardial infarction in a large cohort of emergency department chest pain patients, and to determine whether extended ECG criteria or reciprocal ST depression can improve accuracy. Design: Observational, register-based diagnostic study on the accuracy of ECG criteria for ST-elevation myocardial infarction. Between Jan 2010 and Dec 2014 all patients aged ≥30 years with chest pain who had an ECG recorded within 4 h at two emergency departments in Sweden were included. Exclusion criteria were: ECG with poor technical quality; QRS duration ≥120 ms; ECG signs of left ventricular hypertrophy; or previous coronary artery bypass surgery. Conventional and extended ECG criteria were applied to all patients. The main outcome was acute myocardial infarction (AMI) and an occluded/near-occluded coronary artery at angiography. Results: Finally, 19932 patients were included. Conventional ECG criteria for ST elevation myocardial infarction were fulfilled in 502 patients, and extended criteria in 1249 patients. Sensitivity for conventional ECG criteria in diagnosing AMI with coronary occlusion/near-occlusion was 17%, specificity 98% and positive predictive value 12%. Corresponding data for extended ECG criteria were 30%, 94% and 8%. When reciprocal ST depression was added to the criteria, the positive predictive value rose to 24% for the conventional and 23% for the extended criteria. Conclusions: In unselected chest pain patients at the emergency department, the diagnostic yield of both conventional and extended ECG criteria for ST-elevation myocardial infarction is low. The PPV can be increased by also considering reciprocal ST depression.
Introduction
In patients with suspected ST elevation myocardial infarction (STEMI), i.e. acute myocardial infarction (AMI) due to coronary occlusion, treatment decisions must often be made before cardiac biomarkers are available. These decisions are usually based on electrocardiographic (ECG) criteria for STEMI, i.e. significant ST elevation in at least two anatomically contiguous leads [Citation1].
The diagnostic accuracy of STEMI ECG criteria in the prehospital setting has been described previously. An early meta-analysis of the ECG criteria for STEMI in prehospital chest pain patients showed a fairly high sensitivity of 68% and an excellent specificity of 97% for detecting AMI [Citation2 but a modern study in the same setting reported lower diagnostic accuracy [Citation3].
STEMI incidence at the emergency department (ED) is declining [Citation4] at least partly due to an increased direct transport of STEMI patients to the catherization laboratory, bypassing the ED. In the ED, besides a declining presence of STEMI patients, new high sensitivity troponins for diagnosing AMI and an overall increased number of coronary angiographies [Citation5] may affect the diagnostic yield of ECG criteria in identifying STEMI patients. In general, data on the diagnostic accuracy of STEMI criteria at the ED are scarce [Citation6], and no previous study has analyzed the accuracy of STEMI criteria among chest pain patients at the ED setting in Scandinavia.
In some small studies, sensitivity has been increased by “extending” the ECG STEMI criteria to include leads –aVL, –III, –aVR, –I, –V1, –V2 and –V3 [Citation7,Citation8].
We aimed to evaluate the diagnostic accuracy of ECG amplitude criteria for STEMI in a large cohort of unselected ED chest pain patients, and to analyze the incremental value of reciprocal ST depression and of extended ECG STEMI criteria.
Methods
We performed a retrospective, observational register-based diagnostic study in which all patients admitted to the emergency departments at Skåne University Hospital in Lund and Helsingborg General Hospital (total contingency population >500000) between January 1, 2010 and December 31, 2014 who had an ECG recorded upon admission were eligible for inclusion (the Swedish part of the EXPECT study (Evaluation of Unknown Predictors of Electrocardiographic Changes – a Transnational study) [Citation9]. According to clinical routine at both EDs, an ECG was recorded in all patients who presented with chest pain at the ED. From this population, we included all patients ≥30 years old with a chief complaint of chest pain who had an ECG recorded within 4 h.
We excluded patients with conduction abnormalities (right or left bundle branch block or ventricular pacing) based on QRS duration ≥120 ms, and patients with ECG signs of left ventricular hypertrophy (LVH) using the Sokolow-Lyon criterion [Citation10] (largest S-wave amplitude in V1 or V2 + largest R-wave amplitude in V5 or V6 > 3.5 mV), since STEMI criteria, are not fully applicable in these cases according to the 4th Universal Definition of MI [Citation1]. We also excluded patients with previous coronary artery bypass surgery (CABG) and cases with technically deficient ECGs, defined as the words “unsuitable” or “error” appearing in the computer-based interpretation statement (Glasgow ECG analysis program/Marquette 12SL) for each ECG, or when measurement data were non-biological (e.g. QRS duration ≤60 ms, heart rate <25 beats per minute, or QTc <200 ms or >700 ms). A flow chart of patient inclusion and exclusion is presented in .
Figure 1. Flow chart of patient inclusion and exclusion. Patients were excluded in a stepwise process.
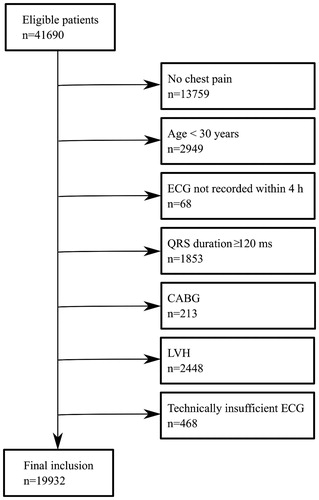
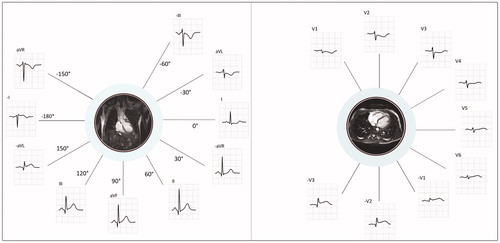
If several ECGs were recorded for a patient, the first ECG was included in the analysis. Automated ECG measurements were used for all analyses. The following STEMI criteria were applied to all patients: ST elevation in at least two contiguous leads of at least 0.1 mV in all leads except V2 – V3, and at least 0.25 mV (males <40 years), 0.2 mV (males ≥40 years), or 0.15 mV (females) in leads V2-V3 [Citation1]. Extended STEMI criteria7,8 which include leads –V1 to –V3 and –aVL, –I, –aVR and –III () were also applied, by which the following contiguous lead pairs were also considered for significant ST elevation (≥0.1 mV): –III/aVL, I/–aVR, –aVR/II, III/–aVL, –aVL/–I, –I/aVR, and –V1/-V2, -V2/-V3. For leads –V2 and –V3 the threshold was set at 0.05 mV [Citation8].
Figure 2. Explanation and basis of the extended STEMI criteria. Limb leads (to the left) and chest leads (to the right) presented in anatomical context.
Left image: The inverted version of lead III (–III) is contiguous and presented adjacently to aVL, the inverted version of aVL (–aVL) is contiguous to lead III, and the inverted version of lead I (–I) is contiguous to both –aVL and aVR. The inverse of aVR (–aVR) is contiguous to lead I and lead II. In this purely illustrative example, significant ST elevation is present in only one of the conventional leads (lead III), but in two contiguous leads when the extended STEMI criteria are applied (III and –aVL).
Right image: In the extended STEMI criteria, the inversion of leads V1, V2 and V3 (–V1, –V2, –V3) are also included in order to cover the lateral aspects of the left ventricle. ECG shows ST depression in V1 – V3, which is equivalent to ST elevation in –V1, –V2 and –V3.
We also analyzed the additive diagnostic value of reciprocal ST depression, i.e. ST depression (≥0.025 mV) in leads II, III or aVF in patients with anterior ST elevation (V2 – V4), or in I, aVL, V2 or V3 in patients with inferior ST elevation (II, aVF, III). A cut-off value of 0.025 mV was suggested in a previous paper [Citation11] and is probably large enough to be observed by “eye balling” the ECG.
Data were extracted from patient records, the regional ECG and clinical chemistry databases, the Swedish pharmacy register, and the Swedish population register. The study database was cross-linked with the Swedish coronary angiography and angioplasty register (SCAAR) [Citation12], which includes (practically) all patients who undergo coronary angiography or percutaneous coronary intervention (PCI) in Sweden, and is a part of the Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) register. During the study period, 1806 patients were sent to coronary angiography due to suspected STEMI directly from the ambulances, from the EDs or from the wards at the two hospitals. Since this study is based on patient data from the ED, only patients who were first admitted to the ED are included in the analysis and the results of the angiographies performed for the remaining patients are unknown.
In this register-based study, a reference standard for the diagnosis of a “true STEMI” could not be reliably defined by a single outcome. Fulfillment of STEMI criteria and a diagnosis of AMI does not necessarily equal STEMI, since patients may have ST elevation for reasons other than STEMI, e.g. early repolarization etc, and have a non-ST elevation myocardial infarction (NSTEMI) despite that amplitude criteria are met. Further, ICD codes do not distinguish between STEMI and NSTEMI [Citation13]. Nor can an occluded coronary artery at angiography define STEMI since occlusion is often a dynamic event [Citation14,Citation15]. We therefore chose to evaluate the accuracy of STEMI criteria for predicting multiple outcomes. The main outcome was a discharge diagnosis of AMI and coronary occlusion or near-occlusion (stenosis ≥90%) at angiography. Secondary outcomes were a) AMI and a coronary occlusion at angiography, b) AMI and coronary stenosis ≥90% at angiography with acute intervention (PCI ad hoc or CABG), c) AMI alone, and d) major adverse cardiac events (MACE) within 30 days. A MACE was defined as AMI, unstable angina, cardiogenic shock, cardiac arrest, ventricular tachycardia/fibrillation, cardiopulmonary resuscitation or death.
The present study was approved by the Regional Ethics Review Board in Lund (Dnr 2015/129 and 2018/705) and is reported according to the STARD guidelines for diagnostic studies [Citation16].
Continuous variables are presented as mean ± standard deviation or as medians and interquartile range, as appropriate. Student’s t-test was used for comparison of means between groups and Mann-Whitney U-test was used for comparison of medians. χ2-test was used for comparison of frequencies between groups. Logistic regression was used to calculate the odds ratio (OR) for ECG criteria in predicting patient outcomes, both unadjusted and adjusted for age, sex, hypertension, hypercholesterolemia, diabetes, AMI, angina pectoris, heart failure, peripheral artery disease, cerebrovascular disease, chronic obstructive pulmonary disease, and renal failure. No power analysis was performed prior to this study. Statistical analysis was performed using SPSS Statistics (Version 26; IBM Corporation, NY, USA).
Results
After exclusions, 19932 ED chest pain patients with an ECG recorded were included in the final analysis (). Baseline characteristics are presented in . Median time from arrival to the ED to ECG recording was 18 min (IQR 26 min). Eighty-nine percent had an ECG recorded within 1 h and 98% within 2 h. Clinical outcomes are summarized in . The diagnostic accuracies of conventional and extended STEMI criteria are described in . Results stratified by age and sex are presented in supplemental Tables A and B.
Table 1. Patient characteristics.
Table 2. Outcomes for patients meeting and not meeting STEMI criteria.
Table 3. Diagnostic accuracy of conventional and extended STEMI criteria.
Among the 19932 patients, 502 (2.5%) fulfilled STEMI criteria, of which 127 (24.9%) were diagnosed with AMI. STEMI criteria were not fulfilled in 19430 patients (97.5%), among which 1211 (6.2%) were diagnosed with AMI ().
A larger proportion of those meeting STEMI criteria underwent coronary angiography (29%) compared to those not meeting STEMI criteria (11%; p < .01). At angiography, an occluded or nearly occluded artery was more commonly detected and intervened upon in patients with AMI who met versus not met STEMI criteria (52% vs. 24%, ad hoc PCI: 83% vs. 69%; p < .01).
Sensitivity and PPV for AMI and coronary occlusion/near-occlusion were low (17% and 12%). Similar values were found for patients who also underwent PCI/CABG (). STEMI criteria fulfillment was associated with an increased likelihood of AMI (OR 5.0 (4.0–6.2)), occluded coronary artery (OR 11.3 (8.3–15.5)), ad hoc PCI (OR 2.0 (1.4–2.9)) and MACE (OR 3.2 (2.6–3.9)) (Supplements Table C).
Among those who met STEMI criteria, patients with AMI and coronary occlusion/near occlusion were slightly older, had higher troponin levels and larger maximum ST elevation than patients without AMI (Supplements Table D). Among patients who fulfilled STEMI criteria but did not undergo coronary angiography (n = 366), only 12 had AMI.
Among the 1249 patients (6.3%) meeting extended STEMI criteria, 217 (17.4%) had a discharge diagnosis of AMI. Among those, an occluded or nearly occluded artery was detected and intervened upon in 351 (28.1%) and 293 (23.5%) cases respectively. For AMI with coronary occlusion/near-occlusion sensitivity increased from 17% to 30%, whereas PPV decreased from 12% to 8%, compared to conventional STEMI criteria.
When reciprocal ST depression was added to STEMI criteria, PPV increased from 12 to 24% for AMI with occlusion/near-occlusion ().
Discussion
The results of the present study indicate that STEMI criteria have a low diagnostic yield in detection of AMI due to acute coronary occlusion in single ECGs recorded in unselected chest pain patients at the ED. In patients with AMI and coronary occlusion/near occlusion, STEMI criteria were met in only 17% of cases, and the PPV was low (12%). Although the PPV was increased when reciprocal ST depression was present, the false positive rate was still high because of the low prevalence. Guidelines state that an ECG should be acquired within 10 min in ED chest pain patients with suspected AMI [Citation17,Citation18] but our results indicate that the vast majority of those meeting STEMI criteria will have neither AMI nor an occluded or nearly occluded coronary artery.
Although several papers have addressed different ECG aspects in STEMI detection (e.g. interpretative skills [Citation19], computer interpretation accuracy [Citation20], culprit artery identification [Citation21], neural networks or automated algorithms [Citation22,Citation23]), few studies have addressed the diagnostic accuracy of the ECG criteria, especially at the ED. Hillinger et al. found that these criteria have low sensitivity (35%) and low PPV (54%) for a STEMI diagnosis at the ED, as adjudicated by two cardiologists [Citation6]. In our study, the sensitivity and PPV for AMI and occlusion/near-occlusion (which may correspond to an adjudicated STEMI diagnosis) were even lower. In addition to our lack of adjudication, the difference might be due to different patient populations. Hillinger et al. only included patients with symptom onset or peak within 12 h, whereas we included patients with chest pain of any duration. Further, 7% of our patients had AMI and 1.3% an occluded coronary artery at angiography compared to 18% and 5.5% in Hillinger’s study.
STEMI criteria are aiming to detect patients with acute coronary occlusion, since these patients may benefit from early revascularization. In our study, a large proportion of patients (24%) with AMI who did not meet STEMI criteria had an occluded/near-occluded coronary artery at coronary angiography, similar to previous reports of the occlusion rate in NSTEMI patients [Citation24,Citation25]. This highlights the importance of finding other ECG criteria to detect patients with acute coronary occlusion.
Extending STEMI criteria or adding reciprocal ST depression improved the diagnostic accuracy less than expected. With the use of extended STEMI criteria, 44 additional patients with AMI and coronary occlusion/near-occlusion could be detected (104 vs. 60 patients), i.e. 73% more patients, but this was accomplished at a cost of more false positives. To detect one additional patient with AMI and coronary occlusion/near-occlusion, 16 more patients would be false positive.
Reciprocal ST-segment changes are known to be valuable in the differential diagnosis of ST elevation [Citation11,Citation26–28] and in the present study, reciprocal ST depression improved the prediction of AMI with coronary occlusion/near occlusion. While sensitivity was lower, extended criteria and reciprocal ST-segment changes combined decreased the number of false positives, compared to STEMI criteria alone. STEMI criteria alone detected 20% more patients with AMI and occlusion/near occlusion, than the combination of extended STEMI criteria and reciprocal ST changes, but the number of false positives was more than twice as large (442 vs. 168). NPV was high for both conventional and extended STEMI criteria, due to the low AMI prevalence at the ED and did not change by adding reciprocal ST depression.
The causes behind the low diagnostic yield of the ECG criteria for STEMI in ED patients are probably multiple. First, ECG interpretation typically includes more than evaluating simple ST amplitude criteria. In addition to reciprocal ST depression, the identification of PR depression [Citation28,Citation29] QRS changes [Citation28,Citation30] or STEMI-equivalent patterns [Citation31,Citation32] are diagnostically helpful. Second, absence of ST elevation is not equivalent to absence of severe ischemic heart disease. Coronary artery disease in need of acute reperfusion can occur without ST elevation, e.g. in left main stenosis or subtotal LAD occlusion [Citation33,Citation34] Culprit vessel patency may change rapidly so that a patient with occlusion and spontaneous reperfusion may be classified as NSTEMI [Citation32]. Third, partly because of ED bypass, there is a low prevalence of patients with true STEMI at the ED. Many patients instead have ST elevation from non-ischemic causes, e.g. pericarditis [Citation35], LVH [Citation36] and early repolarization syndrome [Citation37]. Also, many ED patients have episodical chest pain, while the guidelines recommend that ECG criteria for STEMI should be applied to patients with persistent chest pain [Citation17]. In clinical practice, however, a STEMI diagnosis is initially considered in every ED chest pain patient, since the characteristics of chest pain is not a good predictor of AMI [Citation38,Citation39].
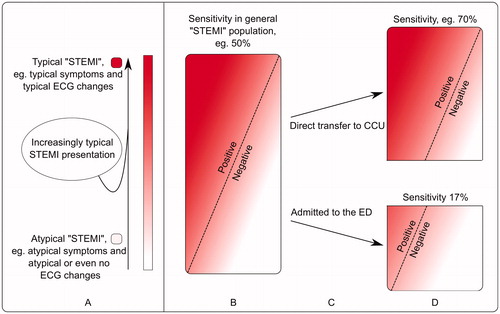
When a large proportion of STEMI patients are directed to the coronary care unit instead of the ED, it is likely that the patients who turn up at the ED have a more atypical presentation, for example atypical symptoms or atypical ECG changes. This is a likely explanation for the observed low sensitivity of STEMI criteria. Although sensitivity and specificity are not generally related to the prevalence of disease (as opposed to PPV and NPV), they can still be affected by a difference in attributes of the patients. When patients with acute coronary occlusion who present with obvious ST elevation are triaged for acute coronary angiography and bypass the ED, the proportion of patients with acute coronary occlusion without obvious ST-segment changes likely becomes larger at the ED, compared to the entire population of patients with acute coronary occlusion (). This negatively affects sensitivity of the STEMI criteria.
Figure 3. Schematic explanation of how sensitivity of STEMI criteria is affected in different populations.
Although sensitivity and specificity are not related to the prevalence of disease (as opposed to PPV and NPV), they can still be affected by a difference in attributes of the patients.
A. The entire population of patients with acute coronary occlusion is heterogeneous and patients may present with either typical ECG changes and typical symptoms on one end of the spectrum, or with atypical symptoms and atypical ECG changes on the other. In this illustration, patients with typical presentation are represented by an intense red color and patients with atypical presentation represented by pink/white colors.
B: The rectangle represents all patients with acute coronary occlusion – here referred to as the general “STEMI” population. STEMI criteria have been assigned a hypothetical sensitivity, 50%, for the detection of acute coronary occlusion. Patients to the left of the dashed line meet STEMI criteria (positive), while patients to the right of the dashed line do not (negative).
C: Patients with typical symptoms and/or typical ECG changes (more red) are more likely to be directed to the coronary care unit (CCU) than those with atypical presentation (pink/white), who instead are more likely to present at the emergency department (ED).
D: Since atypical ECG changes are more common among those patients who present at the ED, STEMI criteria will not detect as many patients with acute coronary occlusion as in the population in B, i.e. sensitivity decreases.
This is the largest study evaluating the diagnostic yield of STEMI amplitude criteria in the ED so far. Our findings, together with those of Hillinger et al. [Citation6], indicate a need for improved ECG criteria in the detection of ED chest pain patients needing acute coronary reperfusion. Automated diagnostic aids using algorithms derived from body-surface potential mapping techniques [Citation40] or using a vectorcardiographic approach [Citation23] have shown promising results, but validation studies in clinical populations are lacking.
Limitations
Since only a minority of the patients underwent coronary angiography, our results need to be interpreted with caution. To overcome the limitation of discrepant results of the coronary angiography and ECG findings of acute coronary occlusion, we analyzed both occluded and near-occluded arteries as outcomes.
Patients with increased QRS duration were excluded from this study since STEMI criteria are not fully applicable to patients with left or right bundle branch block or ventricular pacing. It is possible that exclusion based on QRS duration erroneously excluded some cases where STEMI criteria would have been applicable, i.e. patients with increased QRS duration not due to bundle branch block, LVH or ventricular pacing. AMI prevalence was indeed higher among the excluded patients (12%) than in the study group (7%), but only 2% of these patients had AMI and an occluded/nearly occluded coronary artery, which was similar to the study population.
ECG was registered as a part of routine care in any patient with a complaint of chest pain, i.e. the study likely included patients for which STEMI is an unlikely differential diagnosis, but the number of these patients is unknown. Only single ECGs from the ED were analyzed, while in routine care repeated ECGs are often recorded. Information whether the chest pain was persistent or not, and about symptom duration is lacking, which is a limitation to the study. Also, all types of chest pain were included, i.e. there was no differentiation of typical, atypical or non-anginal pain. The findings in the present study clearly indicate that management decisions must include more information than STEMI criteria fulfillment and acute chest pain, such as other ECG findings (PR depression, QRS changes, reciprocal ST-segment changes, ST-elevation morphology), other diagnostic findings, clinical history as well as the entire gestalt. In this study, most patients who fulfilled STEMI criteria did not undergo coronary angiography, indicating that clinical decision making is indeed based on more than STEMI criteria fulfillment. Even among those who met STEMI criteria and underwent angiography, only half of them had AMI and occlusion/near-occlusion. The results from this study indicate that STEMI criteria alone cannot be used as inclusion criterion in STEMI trials, for example.
In our study, we aimed to evaluate the accuracy of the ECG STEMI criteria in the specific setting of the ED, and our results may not be generalizable to other settings or ED settings with a different proportion of STEMI ED bypass.
The validity of AMI as an outcome in the context of STEMI is questionable, since AMI includes NSTEMI diagnoses. However, AMI as an outcome is less affected by verification bias since it does not require angiographical data. Seventy-five percent of ED chest pain patients who fulfilled STEMI criteria did not have AMI, which is important information to the ED clinician.
Computerized ECG measurements were used in the analysis. We thereby limited the variability of the measurements and avoided human error but may also in some cases have introduced errors. The use of computerized measurements may differ from manually defined ST-J amplitudes. Eskola et al. found slightly larger amplitudes for human measurements compared to computerized measurements [Citation41]. This may have led to slight underestimation of sensitivity and overestimation of specificity compared to if human measurements would have been used.
Conclusion
We found that the diagnostic yield of ECG criteria for STEMI in unselected ED chest pain patients is low. A minority of patients with acute coronary occlusion and AMI met STEMI criteria, and most patients meeting STEMI criteria had neither coronary occlusion nor AMI. Future studies to improve the ECG detection of ED patients with AMI due to acute coronary occlusion are warranted.
Supplemental Material
Download MS Word (34.1 KB)Acknowledgements
We thank Jonas Carlson for excellent technical assistance in handling the ECG database.
Disclosure statement
TL, OP, MB, JLF, PGP and UE have nothing to disclose. MC and HE have received consultancy fees for MRI core lab services in multicenter trials from Imacor, ATL is funded by an unrestricted grant from the philanthropic fund TRYG-Foundation given to University of Southern Denmark.
Additional information
Funding
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Eur Heart J. 2019;40(3):237–269.
- Ioannidis JP, Salem D, Chew PW, et al. Accuracy and clinical effect of out-of-hospital electrocardiography in the diagnosis of acute cardiac ischemia: a meta-analysis. Ann Emerg Med. 2001;37(5):461–470.
- Thang ND, Sundstrom BW, Karlsson T, et al. ECG signs of acute myocardial ischemia in the prehospital setting of a suspected acute coronary syndrome and its association with outcomes. Am J Emerg Med. 2014;32(6):601–605.
- Ward MJ, Kripalani S, Zhu Y, et al. Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. Am J Cardiol. 2015;115(2):167–170.
- Fokkema ML, James SK, Albertsson P, et al. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J Am Coll Cardiol. 2013;61(12):1222–1230.
- Hillinger P, Strebel I, Abächerli R, et al. Prospective validation of current quantitative electrocardiographic criteria for ST-elevation myocardial infarction. Int J Cardiol. 2019;292:1–12.
- Martin TN, Groenning BA, Murray HM, et al. ST-segment deviation analysis of the admission 12-lead electrocardiogram as an aid to early diagnosis of acute myocardial infarction with a cardiac magnetic resonance imaging gold standard. J Am Coll Cardiol. 2007;50(11):1021–1028.
- Perron A, Lim T, Pahlm-Webb U, et al. Maximal increase in sensitivity with minimal loss of specificity for diagnosis of acute coronary occlusion achieved by sequentially adding leads from the 24-lead electrocardiogram to the orderly sequenced 12-lead electrocardiogram. J Electrocardiol. 2007;40(6):463–469.
- Hansen TG, Pottegård A, Brandes A, et al. New-onset atrial fibrillation among patients with infection in the emergency department: a multicentre cohort study of one-year stroke risk. Am J Med. 2019;133(3):352.e3–359.e3.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161–186.
- Bischof JE, Worrall C, Thompson P, et al. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016;34(2):149–154.
- Sarno G, Lagerqvist B, Frobert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of 'new-generation' drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2012;33(5):606–613.
- Alexandrescu R, Bottle A, Jarman B, et al. Current ICD10 codes are insufficient to clearly distinguish acute myocardial infarction type: a descriptive study. BMC Health Serv Res. 2013;13:468.
- Chia S, Senatore F, Raffel OC, et al. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008;1(4):415–423.
- Nikus K, Pahlm O, Wagner G, et al. Electrocardiographic classification of acute coronary syndromes: a review by a committee of the International Society for Holter and Non-Invasive Electrocardiology. J Electrocardiol. 2010;43(2):91–103.
- Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799.
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177.
- Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
- Veronese G, Germini F, Ingrassia S, et al. Emergency physician accuracy in interpreting electrocardiograms with potential ST-segment elevation myocardial infarction: is it enough? Acute Card Care. 2016;18(1):7–10.
- Garvey JL, Zegre-Hemsey J, Gregg R, et al. Electrocardiographic diagnosis of ST segment elevation myocardial infarction: an evaluation of three automated interpretation algorithms. J Electrocardiol. 2016;49(5):728–732.
- Huang X, Ramdhany SK, Zhang Y, et al. New ST-segment algorithms to determine culprit artery location in acute inferior myocardial infarction. Am J Emerg Med. 2016;34(9):1772–1778.
- Forberg JL, Khoshnood A, Green M, et al. An artificial neural network to safely reduce the number of ambulance ECGs transmitted for physician assessment in a system with prehospital detection of ST elevation myocardial infarction. Scand J Trauma Resusc Emerg Med. 2012;20:8.
- Lindow T, Pahlm O, Olson CW, et al. Diagnostic accuracy of the Electrocardiographic Decision Support - Myocardial Ischaemia (EDS-MI) algorithm in detection of acute coronary occlusion. Eur Heart J Acute Cardiovasc Care. 2020;9(1_Suppl):13–25.
- Wang TY, Zhang M, Fu Y, et al. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am Heart J. 2009;157(4):716–723.
- Pride YB, Tung P, Mohanavelu S, et al. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy. JACC Cardiovasc Interv. 2010;3(8):806–811.
- Chung SL, Lei MH, Chen CC, et al. Characteristics and prognosis in patients with false-positive ST-elevation myocardial infarction in the ED. Am J Emerg Med. 2013;31(5):825–829.
- Nfor T, Kostopoulos L, Hashim H, et al. Identifying false-positive ST-elevation myocardial infarction in emergency department patients. J Emerg Med. 2012;43(4):561–567.
- Lindow T, Pahlm O, Khoshnood A, et al. Electrocardiographic changes in the differentiation of ischemic and non-ischemic ST elevation. Scand Cardiovasc J. 2020;54(2):100–107.
- Porela P, Kyto V, Nikus K, Eskola M, et al. PR depression is useful in the differential diagnosis of myopericarditis and ST elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17(2):141–145.
- Lee DH, Walsh B, Smith SW. Terminal QRS distortion is present in anterior myocardial infarction but absent in early repolarization. Am J Emerg Med. 2016;34(11):2182–2185.
- Wall J, White LD, Lee A. Novel ECG changes in acute coronary syndromes. Would improvement in the recognition of 'STEMI-equivalents' affect time until reperfusion? Intern Emerg Med. 2018;13(2):243–249.
- Nikus K, Birnbaum Y, Eskola M, et al. Updated electrocardiographic classification of acute coronary syndromes. Curr Cardiol Rev. 2014;10(3):229–236.
- Yamaji H, Iwasaki K, Kusachi S, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol. 2001;38(5):1348–1354.
- de Winter RJ, Verouden NJW, Wellens HJJ, et al. Interventional Cardiology Group of the Academic Medical C. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359(19):2071–2073.
- Bosson N, Sanko S, Stickney RE, et al. Causes of prehospital misinterpretations of ST elevation myocardial infarction. Prehosp Emerg Care. 2017;21(3):283–290.
- McCabe JM, Armstrong EJ, Kulkarni A, et al. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention-capable centers: a report from the Activate-SF registry. Arch Intern Med. 2012;172(11):864–871.
- Klatsky AL, Oehm R, Cooper RA, et al. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115(3):171–177.
- Mokhtari A, Dryver E, Soderholm M, et al. Diagnostic values of chest pain history, ECG, troponin and clinical gestalt in patients with chest pain and potential acute coronary syndrome assessed in the emergency department. Springerplus. 2015;4:219
- Fanaroff AC, Rymer JA, Goldstein SA, et al. Does this patient with chest pain have acute coronary syndrome?: the rational clinical examination systematic review. JAMA. 2015;314(18):1955–1965.
- Wang JJ, Title LM, Martin TN, et al. Validation of improved vessel-specific leads (VSLs) for detecting acute myocardial ischemia. J Electrocardiol. 2015;48(6):1032–1039.
- Eskola MJ, Nikus KC, Voipio-Pulkki L-M, et al. Comparative accuracy of manual versus computerized electrocardiographic measurement of J-, ST- and T-wave deviations in patients with acute coronary syndrome. Am J Cardiol. 2005;96(11):1584–1588.
