Abstract
Objective
Coronary artery calcification (CAC) is one of the paramount hurdles for percutaneous coronary intervention (PCI) since it impedes stent delivery and complete expansion. This study intended to evaluate the short-term clinical and procedural outcomes comparing rotational atherectomy (RA) and orbital atherectomy (OA) in patients with heavily calcified coronary lesions undergoing PCI. Design: This systematic review and meta-analysis included all head-to-head published comparisons of coronary RA versus OA. Procedural endpoints and post-procedural clinical outcomes (30 days/in-hospital), were compared. RevMan 5.3 software was used for data analysis. Results: Seven retrospective observational investigations with a total of 4623 patients, including 3203 patients in the RA group and 1420 patients in the OA group, were incorporated. Compared with OA, the RA group was associated with a higher incidence of myocardial infarction at short-term follow-up (OR: 1.56, 95% CI: 1.07–2.29, p = .02, I2 = 0%). No difference was noted among other short-term post-procedural clinical outcomes including all-cause mortality, target vessel revascularization, or major adverse cardiac events. Among procedural complications, RA was associated with reduced coronary artery dissection and arterial perforation. Increased fluoroscopy time was observed in the RA cohort as compared with OA (MD: 4.78, 95% CI: 2.25–7.30, p = .0002, I2 = 80%). Conclusion: RA was associated with fewer vascular complications, but at a cost of higher incidence of myocardial infarction and higher fluoroscopy time compared with OA, at short term follow-up. OA is a safe and effective alternative for the management of CAC.
Introduction
The prevalence of coronary artery calcification (CAC) is increasing in the United States. Advanced age, diabetes, chronic kidney disease, and tobacco use are some of the known contributing factors associated with the increased prevalence of CAC [Citation1]. CAC is substantially underrecognized and underdiagnosed in routine practice, which is supported by the fact that CAC was observed in 40.2% of all lesions with angiography and 82.7% with intravascular ultrasound [Citation2]. CAC precludes optimal outcomes with the percutaneous coronary intervention (PCI). Coronary atherectomy devices modify calcified plaque, and there-in facilitate stent deployment and expansion. Rotational atherectomy (RA) has been widely used for many decades to accomplish that purpose. However, orbital atherectomy (OA) has emanated as a newer alternative technique to achieve lesion preparation before stent implantation. Currently, 2011 American College of Cardiology/American Heart Association PCI guidelines endorse a Class IIa recommendation for the use of rotational atherectomy (RA) for the treatment of fibrotic or heavily calcified atheromatous plaques that cannot be crossed by a balloon catheter or adequately dilated before stent implantation (level of evidence C) [Citation3]. However, guidelines did not recommend the routine use of RA (class III and level of evidence A) and do not incorporate the concept of routine lesion preparation for calcified lesions [Citation3]. The ORBIT II trial has shown the efficacy and safety of OA up to 3 years, including low rates of target lesion revascularization (TLR) [Citation4,Citation5]. There has been no large-scale randomized controlled trial that has compared the two atherectomy devices. Observational studies comparing RA and OA are limited, and the results were conflicting [Citation6–9]. Here, we have performed an updated systematic review and meta-analysis comparing RA with OA concerning procedural characteristics and short-term (30 days/in-hospital) clinical outcomes.
Methods
A systematic review of the published data was conducted following the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) statement [Citation10]. A systematic search was performed covering the period from inception to September 2020. Relevant studies were identified from the Medline, Scopus, Cochrane, and EMBASE databases, using the following keywords: “atherectomy,” “orbital atherectomy,” “coronary artery calcification,” “calcified coronaries,” “coronary atherectomy,” and “rotational atherectomy.” (Supplementary Table 1) Two investigators KP and MM, independently screened the yielded articles, using the following criteria: (i) the publication was an original full-length English article in a peer-reviewed journal; (ii) results were reported for patients comparing RA vs. OA for CAC; (iii) the study population consisted of ≥30 patients; and (iv) procedural and clinical outcomes (30 days/in-hospital) were reported.
During the initial screening, the titles and abstracts were reviewed. A second in-depth screening of the full-text articles was performed. In a third screening, the study location and patient inclusion periods were compared to assess whether studies reported results from the same patient population. Sub-analyses of included studies were excluded to avoid counting patient events multiple times. Conflicts between investigators were resolved by discussion. Reference lists from included studies were checked to ensure that no potentially relevant studies were missed. From each study, the following patient baseline characteristics were extracted: age, gender, race, and comorbidities (diabetes mellitus, hypertension, dyslipidemia, smoker, left ventricular ejection fraction, prior myocardial infarction, prior PCI, prior coronary artery bypass graft (CABG)). Study characteristics obtained were: year, country, and the number of centers where studies were conducted. The following procedural endpoints were evaluated: dissection, perforation, tamponade, slow flow/no-reflow, total fluoroscopy time, and total contrast volume used. Short-term (30 days/in-hospital) post-procedure clinical outcomes evaluated were: myocardial infarction (MI), all-cause mortality, target vessel revascularization (TVR), and major adverse cardiac events (MACE), which included the composite of MI, all-cause mortality, and TVR.
Mantel Haenszel method with random-effects models and the inverse variance method with DerSimonian and Laird estimator of tau2 were used to pool categorical and continuous variables, respectively. The odds ratio was calculated for categorical variables and the mean difference was calculated for continuous variables. Heterogeneity across studies was explored using the Cochran Q and I2 statistics, where low values correspond to low heterogeneity. A p-value <.05 was considered statistically significant. Statistical analyses were performed using RevMan 5.3.
Results
illustrates the flow chart of the study selection. A total of 878 articles were reviewed, and a total of seven studies met our inclusion criteria for the analysis. All studies were retrospective observational investigations and are summarized in . The baseline characteristics of patients are presented in . The total sample size was 4623 patients ranging from 117 to 1149 patients per study. A total of 3203 and 1420 patients were included in the RA and OA groups, respectively. The patients were predominately male with mean age and body mass index ranging from 61 to 74 years and 28.1 to 30 kg/m2, respectively; 93.40% had hypertension, 90.03% had hyperlipidemia, 63.76% had diabetes mellitus, 42.66% had prior MI, 44.33% had prior PCI, 32.2% had prior CABG. The distribution of co-morbidities across studies were similar, however, the indication for PCI varied considerably among studies and had been elaborated in .
Figure 1. Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) study selection flow chart.
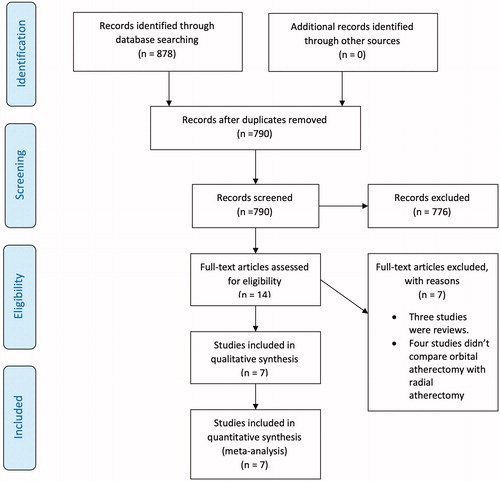
Table 1. Design of included studies.
Table 2. Baseline characteristics of included studies.
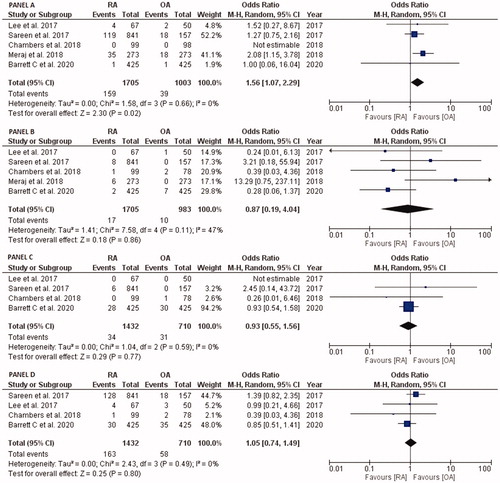
As shown in , five studies reported 30 days/in-hospital clinical outcomes of MI (1705 patients in RA group and 1003 in OA group) and all-cause mortality (1705 patients in RA group and 983 in OA group) whereas four studies described TVR and MACE, including 1432 and 710 patients in RA and OA group, respectively. The odds ratio of 30 days/in-hospital MI with RA compared with OA was OR: 1.56, 95% CI: 1.07–2.29, p = .02, I2 = 0% (Panel A), all-cause mortality was OR: 0.87, 95% CI: 0.19–4.04, p = .86, I2 = 47% (Panel B), TVR was OR: 0.93, 95% CI: 0.55–1.56, p = .77, I2 = 0% (Panel C), and MACE was OR: 1.05, 95% CI: 0.74–1.49, p = .80, I2 = 0% (Panel D).
Figure 2. Forest plots comparing 30 day/in-hospital clinical outcomes of RA versus OA, PANEL A: myocardial infarction, PANEL B: all-cause mortality, PANEL C: target-vessel revascularization, and PANEL D: major adverse cardiac events. Abbreviations: OA, orbital atherectomy; RA, rotational atherectomy.
explains procedural complications. Six studies (2688 in RA group and 1156 in OA group) reported coronary artery dissection comparing RA with OA and a pooled OR: 0.41, 95% CI: 0.20–0.87, p = .02, I2 = 16% (Panel A) while five studies (2571 in RA group and 1089 in OA group) reported arterial perforation comparing RA with OA and a pooled OR: 0.38, 95% CI: 0.15–0.98, p = .04, I2 = 26% (Panel B). Four studies describing cardiac tamponade (2,146 RA patients and 664 OA patients) reported no difference among both groups with pooled OR: 0.43, 95% CI: 0.11–1.69, p = .23, I2 = 0% (Panel C). Similarly, post-procedure slow flow/no-reflow was described in five studies (2,397 in RA and 894 in OA), and it did not show any difference among both groups (OR = 0.95, 95% CI: 0.53–1.73, p = .87, I2 = 0%) (Panel D).
Figure 3. Forest plots comparing procedural outcomes of RA versus OA, PANEL A: Coronary artery dissection, PANEL B: arterial perforation, PANEL C cardiac tamponade, PANEL D slow flow/no-reflow, PANEL E: total fluoroscopy time (in minutes), and PANEL F: total contrast volume used (in millimeters). Abbreviations: OA, orbital atherectomy; RA, rotational atherectomy.
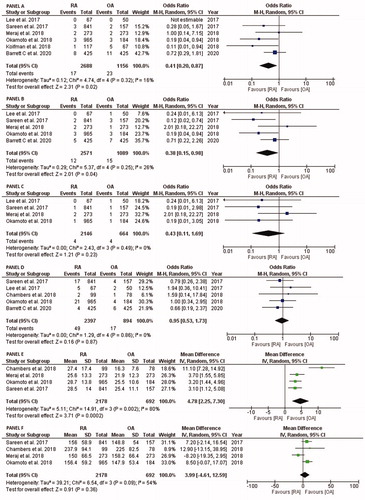
Four studies reported procedural fluoroscopy time and total contrast volume used in 2178 and 692 patients among RA and OA groups, respectively. Compared with OA, RA was consistently associated with higher total fluoroscopy time (MD: 4.78, 95% CI: 2.25–7.30, p = .0002, I2 = 80%) (Panel E). The total contrast volume used did not differ between the groups (MD: 3.99, 95% CI: −4.61 to 12.59, p = .36, I2 = 54%) (Panel F).
Discussion
This updated meta-analysis comparing RA and OA for patients with CAC undergoing PCI demonstrates, short-term (30 days/in-hospital) clinical outcomes and angiographic procedural complications were comparable in both arms. RA was associated with a higher incidence of 30 days/in-hospital MI compared with OA. Nonetheless, there was no difference in 30 days/in-hospital all-cause mortality, TVR, or MACE. Despite higher MI incidence with RA, the risk of coronary artery dissection and perforation were lower with RA compared to OA. At the same time, fluoroscopy time was lower in the OA arm as compared with RA. However, heterogeneity was higher for this analysis. We conclude that both RA and OA can be considered as safe and effective modalities (at short term follow-up) for plaque modification in moderate to severely calcified lesions before PCI and stent deployment.
Simple atherosclerotic lesions are managed by balloon angioplasty and stent implantation, with a trend of improving success. More complex atherosclerotic plaques containing moderate to severe calcification imparts additional demur for interventional procedures and are known to carry lower success rates and increased complication rates following PCI compared to non-calcified or mildly calcified lesions [Citation11,Citation12]. Severe calcification also poses technical challenges during PCI, resulting in stent under-expansion, mal-apposition, or the inability to deliver a stent [Citation11]. Additionally, extensive calcification may damage the polymer coating of drug-eluting stents (DES) [Citation13,Citation14] and, therefore, contribute to the high failure rate of DES when implanted into such lesions [Citation15]. OA is the first device approved explicitly for routine lesion preparation of calcified lesions before stent implantation. However, the current guidelines do not mention a recommendation for orbital atherectomy, given that the device approval followed the issuance of the most recent guidelines.
A single-arm prospective pilot study, ORBIT I, assessed the safety and efficacy of OA by delineating acceptable periprocedural complications and 3-year MACE [Citation16,Citation17]. Subsequently, the single-arm prospective multicenter ORBIT II study further demonstrated the safety and efficacy of OA in a larger population [Citation5,Citation18]. However, the ORBIT studies excluded patients with the unprotected left main disease, impaired left ventricular systolic function with ejection fraction less than or equal to 25%, chronic kidney disease unless on dialysis, long diffuse disease (>40mm lesion length), and recent myocardial infarction. Nonetheless, real-world multicenter studies including an all-comer population with severe CAC who underwent orbital atherectomy followed by stenting has found similar results with OA [Citation19,Citation20].
Despite overlapping similarities between RA and OA, there are several significant differences in device and indication for use. In terms of technical aspects, OA is quick to set up and user-friendly because it does not require a nitrogen tank and a foot pedal to activate the device. OA has a table-side, electric-powered motor handle with an on-handle power switch, on-handle speedometer containing options for low speed (80,000 rpm), high speed (120,000 rpm), or GlideAssist (5000 rpm). This attribute of an on-handle speed control assists the physician in treating lesions of vessels with various diameters such as high speed for larger diameter vessels, without requiring tech-assistance to adjust speed nor a need for a change in burr size for larger vessels. More recent iterations of the RA device now include a tableside controller, which improves the usability and setup associated with RA. OA utilizes a standard 1.25 mm crown that spins over a 6 F ViperWire for all cases, while RA uses burrs of different sizes (1.25–2.50 mm), which necessitates a ≥ 7 F catheter for burr sizes ≥1.75 mm. In OA, the crown is mounted eccentrically. It orbits along the periphery of the vessel, expanding elliptically to generate the centrifugal force that leads to the debulking of calcified plaque. OA ablates bidirectionally, which leads to less chance of crown entrapment. There are essential differences in optimal device advancement owing to the different mechanisms of action and technical aspects. RA can often be advanced with a pecking back-and-forth motion, whereas OA should be advanced slow and steady at approximately 1 mm per second. Optical coherence tomography (OCT) demonstrated that OA induces more remarkable modification of plaque, including longer cuts and dissections, particularly in larger lumens. It may cause a lower percentage of stent strut mal-apposition and a trend toward improved stent expansion compared to RA [Citation21]. A prospective multicenter study evaluating the safety and efficacy of a novel Micro Crown OA, designed for use in tighter calcified lesions, showed a similar rate of procedural success and freedom from MACE compared to Classic Crown OA [Citation22].
Previous meta-analyses by Sawant et al. and Goel et al. did not show significant differences in post-procedural MI between RA and OA [Citation23,Citation24]. This present meta-analysis showed a higher incidence of 30 days/in-hospital MI with RA compared with OA. Studies by Lee et al. [Citation25], Sareen et al. [Citation6], and Meraj et al. [Citation7] reported a higher rate of short-term MI associated with RA, which in turn, translated into our meta-analysis. Owing to a single axis rotation in RA, the burr is in constant contact with the plaque, eliciting potential thermal injury, platelet activation [Citation26], and release of particles 5-um in size in a bolus, increasing the risk of thrombus formation and slow or no-reflow [Citation27]. Whereas in OA, a crown is in intermittent contact and continuously releases particles of 2-um in size, accounting for a theoretically lower chance of distal embolization, transient heart block, and a lower rate of slow/no-flow, accounting for low periprocedural MI. A meta-analysis by Sawant et al. showed higher odds of all-cause mortality and MACE with RA compared with OA. However, the analysis was primarily influenced by the results of a single study, which was not propensity-matched [Citation28]. Additionally, the meta-analysis included studies that varied considerably in the follow-up duration. Furthermore, the sample sizes in previous meta-analyses were 2357 [Citation23] and 1872 [Citation24] compared to a total of 4623 in the current study.
Moreover, our study exhibited a lower incidence of arterial dissection and perforation in RA compared with OA, which was similar in the previous meta-analyses [Citation23,Citation24]. Among six studies reporting coronary artery dissection, Sareen et al. [Citation6], Okamoto et al. [Citation28], Koifman et al. [Citation29], and Barrett et al. [Citation9] reported a lower rate of CAD in RA compared with OA. Increased occurrence of arterial perforation was reported by Lee et al. [Citation25], Sareen et al. [Citation6], Okamoto et al. [Citation28], and Barrett et al. [Citation9] This may be explained by elliptically expanding centrifugal force orbiting along the periphery of the vessel [Citation21], leading to higher coronary dissections and perforations. The impact of the operator learning curve and optimal lesion selection on complications with OA is unknown at present. Our study population had lower fluoroscopy time in the OA group compared to the RA group, albeit with higher heterogeneity. The unique attributes associated with OA may ultimately lead to less fluoroscopic procedural time, as shown in this study. This result may be explained by the bidirectional atherectomy action of OA and a single sized burr, making OA more efficient and potentially less time-consuming.
Ultimately, this meta-analysis adds to the existing literature demonstrating the safety of both RA and OA. The safety of contemporary devices supports a paradigm shift from merely debulking and facilitating stent delivery in difficult to cross lesions to routine preparation of calcified lesions to optimize stent results and maximize final stent expansion. Several clinical trials are ongoing evaluating treatment strategies for severe calcified coronary arteries. Most notably, the ECLIPSE trial (NCT03108456), is a landmark multicenter trial randomizing 2,000 patients with severely calcified lesions to either orbital atherectomy or conventional balloon angioplasty before stent implantation. The primary endpoint of the study is target vessel failure, defined as the composite of cardiac death, target vessel-related MI, or ischemia-driven TVR. Moreover, there is an OCT imaging subgroup cohort in which the primary endpoint will compare the final minimal stent area between groups [Citation21,Citation30]. Future data may inform optimal lesion selection for atherectomy devices to minimize procedural complications.
Our study demonstrated a few important limitations. First, all the studies included in the analysis are retrospective and non-randomized. Secondly, the data collection was during a time when experience with OA was in its initial stages. Hence, it might have led to both selection bias for less complex lesions and might have negatively altered the clinical outcomes due to lack of device experience. These confounding factors need to be studied in a prospective and randomized study. Documentation on the calcification in terms of length, arc, and depth was not reported nor analyzed by a core laboratory and was determined based on the operator’s evaluation; hence significant baseline differences in severity of lesion calcification may exist. The decision to select RA or OA was at the operating physicians’ discretion. The overall follow-up length is short as limited data is available beyond 30-day follow-up in studies comparing these two modalities. Future studies are needed to determine if there is a significant difference in long-term rates of target lesion revascularization.
Conclusions
No major difference between RA and OA for patients with CAC undergoing complex PCI was observed in the existing literature, at short term follow-up. Our study revealed that RA is associated with a higher risk of 30 days/in-hospital MI than OA, but was associated with a lower incidence of coronary artery dissection and perforation. However, RA was associated with higher procedural fluoroscopy time. Future studies are needed to delineate differences in long-term outcomes for each device as current literature suggests equipoise in device safety and efficacy.
Authors’ contribution
Rajkumar Doshi, MD, MPH: Conceptualization, methodology, data extraction, visualization, writing- original draft preparation; Samarthkumar Thakkar, MD: Conceptualization, methodology, writing- original draft preparation; Krunalkumar Patel, MD: Conceptualization, methodology, data extraction, writing- original draft preparation; Monil Majmundar, MD: Methodology, data extraction; Evan Shlofmitz, DO: Methodology, Data Extraction And Validation; Ashish Kumar, MBBS: Methodology, software, validation, writing- reviewing and editing; Neelesh Gupta, MD: Methodology, data extraction; Devina Adalja, MBBS: Methodology, data extraction; Harsh P. Patel, MD: Methodology, data extraction; Rajiv Jauhar, MD: Conceptualization, methodology, supervision; Perwaiz Meraj, MD, MPH: Conceptualization, methodology, supervision.
Supplemental Material
Download MS Word (16.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Madhavan MV, Tarigopula M, Mintz GS, et al. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703–1714.
- Wang X, Matsumura M, Mintz GS, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10(8):869–879.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):2574–2609.
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510–518.
- Lee M, Genereux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18(4):261–264.
- Sareen N, Baber U, Aquino M, et al. Mid-term outcomes of consecutive 998 cases of coronary atherectomy in contemporary clinical practice. J Interv Cardiol. 2017;30(4):331–337.
- Meraj PM, Shlofmitz E, Kaplan B, et al. Clinical outcomes of atherectomy prior to percutaneous coronary intervention: a comparison of outcomes following rotational versus orbital atherectomy (COAP-PCI study). J Interv Cardiol. 2018;31(4):478–485.
- Chambers JW, Warner C, Cortez J, et al. Outcomes after atherectomy treatment of severely calcified coronary bifurcation lesions: a single center experience. Cardiovasc Revasc Med. 2019;20(7):569–572.
- Barrett C, Warsavage T, Kovach C, et al. Comparison of rotational and orbital atherectomy for the treatment of calcific coronary lesions: insights from the VA clinical assessment reporting and tracking (CART) program. Catheter Cardiovasc Interv. 2020.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Mosseri M, Satler LF, Pichard AD, et al. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med. 2005;6(4):147–153.
- Genereux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) Trials. J Am Coll Cardiol. 2014;63(18):1845–1854.
- Pinto DS, Stone GW, Ellis SG, et al. Impact of routine angiographic follow-up on the clinical benefits of paclitaxel-eluting stents: results from the TAXUS-IV trial. J Am Coll Cardiol. 2006;48(1):32–36.
- Serruys PW, van Hout B, Bonnier H, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet. 1998;352(9129):673–681.
- Khattab AA, Otto A, Hochadel M, et al. Drug-eluting stents versus bare metal stents following rotational atherectomy for heavily calcified coronary lesions: late angiographic and clinical follow-up results. J Interv Cardiol. 2007;20(2):100–106.
- Parikh K, Chandra P, Choksi N, et al. Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv. 2013;81(7):1134–1139.
- Bhatt P, Parikh P, Patel A, et al. Orbital atherectomy system in treating calcified coronary lesions: 3-year follow-up in first human use study (ORBIT I trial). Cardiovasc Revasc Med. 2014;15(4):204–208.
- Genereux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial). Am J Cardiol. 2015;115(12):1685–1690.
- Lee MS, Shlofmitz E, Kaplan B, et al. Real-world multicenter registry of patients with severe coronary artery calcification undergoing orbital atherectomy. J Interv Cardiol. 2016;29(4):357–362.
- Sturm R, Martinsen BJ, Valle JA, et al. Orbital atherectomy for treatment of complex severely calcified coronary artery lesions: insights from a veterans affairs cohort. Cardiovasc Revasc Med. 2020;21(3):330–333.
- Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86(6):1024–1032.
- Redfors B, Sharma SK, Saito S, Kini AS, et al. Novel micro crown orbital atherectomy for severe lesion calcification: Coronary Orbital Atherectomy System Study (COAST). Circ Cardiovasc Interv. 2020;13(8):e008993.
- Sawant AC, Panchal H, Radadiya D, et al. Comparison of rotational with orbital atherectomy during percutaneous coronary intervention for coronary artery calcification: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2020;21(4):501–507.
- Goel S, Pasam RT, Chava S, et al. Orbital atherectomy versus rotational atherectomy: a systematic review and meta-analysis. Int J Cardiol. 2020;303:16–21.
- Lee MS, Park KW, Shlofmitz E, et al. Comparison of rotational atherectomy versus orbital atherectomy for the treatment of heavily calcified coronary plaques. Am J Cardiol. 2017;119(9):1320–1323.
- Lovik RD, Abraham JP, Sparrow EM. Assessment of possible thermal damage of tissue due to atherectomy by means of a mechanical debulking device. Paper presented at the ASME Summer Bioengineering Conference (SBC2008); 2008 June 25–29; Marco Island, FL.
- Kume T, Okura H, Kawamoto T, et al. Assessment of the histological characteristics of coronary arterial plaque with severe calcification. Circ J. 2007;71(5):643–647.
- Okamoto N, Ueda H, Bhatheja S, et al. Procedural and one-year outcomes of patients treated with orbital and rotational atherectomy with mechanistic insights from optical coherence tomography. EuroIntervention. 2019;14(17):1760–1767.
- Koifman E, Garcia-Garcia HM, Kuku KO, et al. Comparison of the efficacy and safety of orbital and rotational atherectomy in calcified narrowings in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2018;121(8):934–939.
- Shlofmitz E, Shlofmitz R, Lee MS. Orbital atherectomy: a comprehensive review. Interv Cardiol Clin. 2019;8(2):161–171.
