Abstract
Objectives. Urinary albumin excretion is a risk marker for cardiovascular disease (CVD). Studies suggest that urinary orosomucoid may be a more sensitive marker of general endothelial dysfunction than albuminuria. The aim of this population-based cross-sectional study was to examine the associations between urinary orosomucoid to creatinine ratio (UOCR), urinary albumin to creatinine ratio (UACR) and subclinical CVD. Design. From the Tromsø Study (2007/2008), we included all men and women who had measurements of urinary orosomucoid (n = 7181). Among these, 6963 were examined with ultrasound of the right carotid artery and 2245 with echocardiography. We assessed the associations between urinary markers and subclinical CVD measured as intima media thickness of the carotid artery, presence and area of carotid plaque and diastolic dysfunction (DD). UOCR and UACR were dichotomized as upper quartile versus the three lowest. Results. High UOCR, adjusted for UACR, age, cardiovascular risk factors and kidney function, was associated with presence of DD in men (OR: 3.18, 95% CI [1.27, 7.95], p = .013), and presence of plaque (OR: 1.20, 95% CI [1.01, 1.44], p = .038) and intima media thickness in women (OR: 1.34, 95% CI [1.09, 1.65], p = .005). Analyses showed no significant interaction between sex and UOCR for any endpoints. UACR was not significantly associated with DD, but the associations with intima media thickness and plaque were of magnitudes comparable to those observed for UOCR. Conclusions. UOCR was positively associated with subclinical CVD. We need prospective studies to confirm whether UOCR is a clinically useful biomarker and to study possible sex differences.
Introduction
Cardiovascular disease (CVD) is still the number one cause of death globally [Citation1]. Several observational studies, including meta-analyses, in different risk groups have repeatedly confirmed that increased albuminuria is an independent risk factor for mortality. However, the exact mechanisms explaining the link between albuminuria and clinical disease in vessels are not known. Albuminuria represents a dysfunctional filtration barrier, but also depends on tubular cell reabsorption and secretion, and albumin might be added to the urine from the urinary tract as well [Citation2,Citation3]. Thus, albuminuria is unspecific and may represent different pathophysiological processes. Moreover, it has not yet been confirmed that lowering of albuminuria per se reduces the risk of CVD [Citation4,Citation5]. Thus, the clinical relevance of albuminuria measurements in cardiovascular medicine, especially in low and medium risk non-diabetic populations, is unsettled and novel biomarkers that may shed light on the early mechanisms of endothelial dysfunction are needed.
Orosomucoid (molecular weight 41kD, negatively charged) is a nonspecific acute inflammatory protein mainly produced in the liver, but also in endothelial cells and other extra-hepatic tissue [Citation6]. Orosomucoid is found among other plasma proteins as well as glycosaminoglycans and proteoglycans in the endothelial surface layer, a cell coat that covers the luminal side of the endothelium throughout the vasculature. The endothelial surface layer protects the vessel wall from contact with blood constituents and is important for the maintenance of normal glomerular permselectivity. Damage to this layer is believed to be a direct cause of albuminuria and to be involved in the development of atherosclerotic vascular disease [Citation7–10].
Under normal conditions, orosomucoid is present in urine in very low concentrations. The reference range in healthy individuals has not yet been settled, but a recent study based on 72 healthy adolescents and adults (within 10–60 years of age) suggested a reference range for urinary orosomucoid to creatinine ratio (UOCR) between 0.01 and 0.24 mg/mmol, with a median of 0.08 mg/mmol (corresponding to 0.71 mg/g) [Citation11]. This result corresponds to Christansen et al. where they described a reference range for UOCR 0.009–0.17 mg/mmol, median 0.04 mg/mmol (corresponding to 0.36 mg/g) [Citation12]. Observational studies have shown increased levels of urinary orosomucoid in patients with chronic diseases such as chronic heart failure, psoriasis, rheumatoid arthritis and diabetes [Citation13–16], suggesting that urinary orosomucoid excretion (UOE) may be a marker of dysfunctional endothelium and endothelial surface layer. Indeed, studies in patients with type 2 diabetes and normal urinary albumin excretion (UAE) have shown that increased UOE independently predicts cardiovascular mortality [Citation17,Citation18]. Also, a recent study found an association between UOCR, but no other biomarkers, and an increased cardiovascular risk score [Citation19].
Little is known about the relationship between UOE and cardiovascular endpoints in the general population. However, since UOE probably reflects damaged or dysfunctional endothelium at an earlier stage than UAE, this biomarker may be eneralized to aid in early risk stratification in CVD. Measurements of intima media thickness (IMT) as well as plaque in the carotid artery are considered valid markers of generalized arterial injury and predictors of future cardiovascular events [Citation20,Citation21]. Also, left ventricular diastolic dysfunction (DD), which is prevalent in patients with cardiovascular risk factors such as hypertension, obesity and diabetes, is known to be strongly associated with endothelial dysfunction [Citation22,Citation23]. Therefore, we assessed the cross-sectional association between UOE and both carotid arteriopathy and DD in a large cohort from the general population.
Materials and methods
Study population
The Tromsø Study is a population-based, prospective study with repeated health surveys of inhabitants of Tromsø, a municipality in Northern Norway [Citation24]. The scope of the sixth survey (Tromsø 6), conducted in 2007–2008, was to include both new participants and participants from the second visit with more extensive examination in Tromsø 4 (1994–1995). To the first visit of Tromsø 6 all residents aged 40–42 and 60–87, a 10% random sample of individuals aged 30–39 and a 40% random sample of individuals aged 43–59 plus subjects who had attended the second visit of Tromsø 4 (if not already included) were invited. A total of 12 984 subjects (6930 women and 6054 men, 65.7% of the invited population) took part in the survey. A few weeks after the first visit, a subgroup was invited to a second visit with extensive examination. This subgroup was defined by first-visit participants aged 50–62 and 75–84, a 20% random sample in the age 63–74 years and all participants from the second visit of Tromsø 4 (if not already included). A total of 7307 (64% of eligible persons) attended. The participants delivered urinary samples (n = 7181) and underwent ultrasonography of the right carotid artery (n = 7083). Those who had undergone transthoracic echocardiography in Tromsø 4 and/or Tromsø 5 were also invited to a new echocardiography in Tromsø 6, and 2284 participated (96,7%). In the present study, we included all participants who delivered urinary samples (n = 7181) and participated in carotid ultrasound examination (n = 6963) and echocardiography (n = 2245).
The Tromsø Study has been approved by the Regional Committees for Medical and Health Research Ethics and follow the ethical guidelines of the 1975 Declaration of Helsinki. All participants gave their written, informed consent.
Measurements
Two self-administered questionnaires about various health issues, medication and smoking habits were completed at the first visit. Blood pressure (BP) was measured using an automated device (Dinamap Pro care 300 Monitor, GE Healthcare Norway). Three readings on the right upper arm were taken while seated, at 1-minute interval after a 2-min rest. We used the mean of the last two measurements. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg and/or self-reported use of BP lowering drugs. Body mass index (BMI) was calculated as weight in kg divided by the square of the height in meters. Non-fasting serum total cholesterol were analyzed by enzymatic colorimetric method and HbA1c by automated HPLC method (Bio-Rad Laboratories, Munich, Germany). Diabetes was defined as self-reported diabetes and/or self-reported use of anti-diabetic medication and/or HbA1c ≥6.5% (48 mmol/mol). Smoking habits were defined as current smoking or no smoking; the nonsmoking group consisted of never smokers and previous smokers. Blood samples were nonfasting. An enzymatic method was used to analyze serum creatinine (standardized against isotope dilution mass spectroscopy, CREA Plus, Roche Diagnostics, GmbH, Mannheim, Germany). Estimated glomerular filtration rate (eGFR) was calculated applying the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [Citation25].
Urinary samples
At the second visit, all participants delivered fresh morning urinary specimens from three consecutive days. The samples were immediately analyzed for albumin and creatinine (unfrozen urine within 20 h after collection using an ABX PENTRA autoanalyzer Horiba ABX) and kits from ABX Diagnostics (Montpellier, France). About 10 ml of each specimen was frozen at −20 °C and shipped to Randers, Denmark, where they were analyzed for orosomucoid by a highly sensitive EU (Europium)-fluoroimmunosorbent assays using a sandwich technique. Stability studies of urinary orosomucoid have shown stable values despite repeated freeze-thaw cycles [Citation11]. The DELFIA (dissociation-enhanced lanthanide fluorescence immunoassay) system was used for the assay. The method has been described more in detail previously [Citation26,Citation27].
Urinary orosomucoid measurements were available in 7083 participants (4024 women and 3059 men). The concentration was divided by the creatinine concentration of the same specimen, and median values were used. For UOCR, we chose to use the unit mg/g instead of mg/mmol to obtain numerical values in the same range as for urinary albumin to creatinine ratio (UACR) in mg/mmol.
Echocardiography
Echocardiography (Acuson Sequoia C512 Ultrasound System) included assessment of systolic and diastolic left ventricular function indices, left atrial (LA) size and ventricular wall thickness. Details about the procedure and its reproducibility have been published previously [Citation28].
The definition of DD has changed over the last years, and we applied a modified version of the 2016 recommendations from the American Society of Echocardiography and European Association of Cardiovascular Imaging [Citation29]. In accordance with the recommendations, we used different set of criteria depending on whether the ejection fraction (EF) was normal (defined as ≥50%) or reduced. Since our data did not include measurements of the right ventricle, we modified the definition accordingly, and we set two of three criteria as diagnostic for DD. This covers the old definition of DD which gave prevalence ten times higher than the current.
We used left atrial (LA) diameter/surface area (Lad BSA) as an index of atrial size since we were missing volume measurements (Lad BSA >2.8 cm/m2 is equivalent to LA volume index >34 ml/min/m2). Since EF <40% will cause high probability of components of DD, we excluded those participants (n = 12).
Plaque and intima media thickness in right carotid
At the second visit of Tromsø 6, 7083 participants underwent a full carotid scan with measurements of IMT and plaque characteristics with GE Health Care Vivid 7 duplex scanners with M12L transducers (5.6–14 MHz linear transducer). Trained sonographers performed the examinations. IMT was measured in the near and far wall of the distal right common carotid artery and in the far wall of the bifurcation [Citation28], and the average of the mean value of three separate measurements in each of these segments is presented as mean IMT. The presence of plaque (n = 3290) was assessed in the near and far walls of the distal common carotid artery, the carotid bifurcation and the internal carotid artery. A plaque was defined as a localized thickening of the vessel wall of more than 50% compared with the adjacent IMT. Plaque area was calculated by outlining each area, and the sum of all plaque areas in the six arterial segments was defined as total plaque area (TPA). The distribution of TPA was skewed, and TPA was square root transformed to approximate normal distribution. The inter- and intraobserver reproducibility has been published previously [Citation30,Citation31].
Statistical analysis
Statistical software package IBM SPSS. Version 26.0 (Armonk, NY, USA) was used for all data analysis. Due to established sex differences in cardiometabolic pathophysiology [Citation32], we chose to run sex specific analyses. Sex-stratified cohort characteristics were given for the entire cohort and for the subjects who underwent echocardiography. All variables were normally distributed and listed as mean (standard deviation [SD]), except for UACR and UOCR, which were listed as median (interquartile range). To check for sex differences within characteristics, independent sample Student`s t-test, Chi-square tests and Mann–Whitney U-tests were applied as appropriate. We tested the bivariate correlations between the urinary biomarkers and the endpoints as well as covariates. To study the effect of creatinine concentration in the urine we analyzed the correlations both with and without adjustment for creatinine. Because of the skewed distribution of the urinary biomarkers, Spearman’s correlation was used. IMT was categorized into quartiles, and the upper quartile was defined as subclinical cardiovascular disease. TPA was categorized as no plaque and in tertile in those with TPA > 0mm3, and comparisons were made between the upper tertile and the other categories combined.
We assessed the associations between the urinary biomarkers and the endpoints DD, IMT, TPA and presence of plaque applying different multiple logistic regression models and correlation. The urinary biomarkers were entered into separate models either as continuous variables (squared transformed) or as dichotomous variables with the highest quartile versus the three lowest. Sex-specific quartiles were used. Both urinary markers were included and adjusted for each other. The simplest models were age adjusted only, whereas the fully adjusted models included hypertension, diabetes, BMI, current smoking, serum cholesterol and eGFR as covariates. We tested for interaction between the urinary biomarkers and sex in all the regression models, and area under the receiver operating characteristic (ROC) curves (AUC) were tested for the full model. A two-sided p-value of <0.05 was considered statistically significant.
Results
Cohort characteristics
Baseline characteristics of all participants with UOCR measurements (n = 7181) are listed in . Women and men did not differ significantly in age, kidney function and systolic BP. Men had a higher prevalence of diabetes and hypertension, higher BMI and HbA1c, whereas women had higher cholesterol and were more frequently smokers. Median UACR was significantly higher in women than in men, whereas the opposite was found for UOCR. Without adjustment for creatinine, median UOE and UAE were both higher in men than in women.
Table 1. Study population characteristics. The Tromsø Study 2007-08, n = 7181.
Almost all participants with UOCR measurements also underwent carotid scan (n = 6963; 98% of eligible). The baseline characteristics were essentially unchanged when the 218 subjects without carotid scanning were excluded.
Among the subjects with echocardiography and UOCR measurement (n = 2245), 12 subjects had EF <40% and were therefore excluded from further analyses (n = 2233). In this subcohort, 57 participants (31 women and 26 men; p for sex difference .89) met the criteria for DD. In , we show baseline characteristics for the echocardiography subcohort, compared to those who did not have this examination. The echo cohort was selected to this examination based on their participation in earlier Tromsø studies. Mean age, systolic BP and HbA1c were significantly higher in those who underwent echocardiography than in the rest of the cohort, and eGFR was lower (). In addition, men were significantly older than women (65.9 ± 10.3 versus 64.6 ± 11.5 years; p = 0.003) and had a higher median UOCR (men 0.59 (0.27, 1.53) mg/g versus women 0.41 (0.23, 0.86) mg/g; p = <0.001) (Table S1).
Table 2. Comparing basic characteristics from the whole cohort to the subgroup with echocardiography.
Correlation between urinary biomarkers and the subclinical endpoints
Bivariate correlations between the urinary biomarkers and all covariates were highly significant. The r-values are presented in . Since the same creatinine value was used in the denominator of both UOCR and UACR of each sample, we assessed the urinary biomarkers both with and without adjustment for creatinine. UOE was strongly correlated to UOCR, r = 0.89, as was UAE to UACR (r = 0.86). There was a moderate correlation between UOE and UAE (r = 0.44), as for UOCR and UACR (r = 0.43). Age had a stronger correlation to the creatinine adjusted markers than to UOE and UAE, respectively. The Spearman’s r for correlations between UOCR and DD as well as the carotid measurements were numerically higher than the corresponding correlation coefficients between UACR and the same endpoints.
Table 3. Spearman’s Correlation between urinary biomarkers and covariates.
Associations between UOCR, UACR and DD
shows that median UOCR was higher in participants with DD compared to participants with normal diastolic function (p = .02). The same phenomenon was not observed for UACR (p = .079). In multivariable logistic regression analysis UOCR entered as a continuous variable was not significantly associated with DD. However, in men, the squared term of UOCR was a significant predictor (OR per one unit increase 1.59, 95%CI [1.10, 2.28], p = .013), suggesting a non-linear association between UOCR and DD.
Figure 1. Urinary biomarkers in persons with and without diastolic dysfunction (error bars with 95% CI).
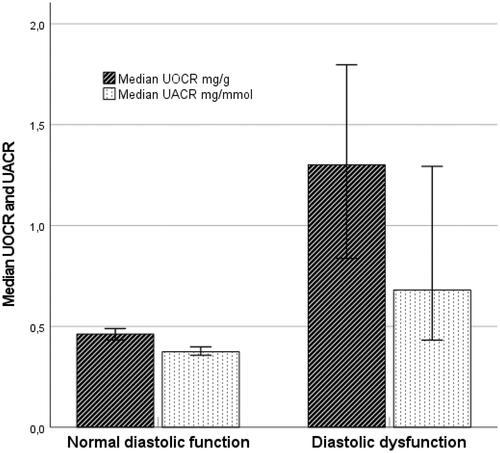
We found a strong association between age-adjusted UOCR entered as a dichotomous variable (upper quartile versus the three lower) and DD in men (OR 3.78, 95%CI [1.64, 8.69], p = .002) (). The association was essentially unchanged after adjustment for UACR age, hypertension, cholesterol, eGFR, BMI, diabetes and current smoking (OR 3.18, 95% CI [1.27, 7.95], p = .013). We did not find association between UOCR and DD in women (OR: 1.39, 95% CI [0.59, 3.28], p = .453). However, there was no significant interaction between sex and UOCR. UACR, on the other hand, was not associated with DD for either men or women when adjusted for UOCR and the same risk factors. Analysis in show ROC AUCs for DD, model 1 with full adjustments and model 2 without UOCR in quartiles. AUC difference shows a borderline significantly increased AUC value for men (p = .071), but not for women (p = .456).
Table 4. Odds ratio for having subclinical cardiovascular disease.
Table 5. Area under the ROC curve (AUC).
Associations between UOCR, UACR and carotid arteriopathy
UOCR squared was significantly associated with IMT in both sexes and with the presence of plaque in women in fully adjusted models (data not shown). Age-adjusted UOCR in the upper quartile was associated with presence of carotid plaque and IMT in both women and men (). There was no significant interaction between sex and UOCR or UACR in neither of the models. With full adjustment for cardiovascular risk factors and kidney function, UOCR in upper quartile remained significantly associated with presence of plaque in women (OR 1.20, 95% CI [1.01, 1.44], p = 0.038). In men the association was numerically similar and of borderline significance. IMT was significantly associated with both urinary markers in women, but not in men. Age-adjusted TPA in the upper tertile was associated with UOCR and UACR in women and men. This association remained significant in fully adjusted models (Table S2). AUC values from ROC for carotid arteriopathy presented in and S3 show significant increase when adding UOCR to the model for TPA in men (p = .022), and borderline significant increase for IMT in women (p = .062).
Discussion
In the present study, there were significant and independent associations between UOCR, a urinary biomarker of endothelial dysfunction, and subclinical markers of arterial injury and early CVD. The associations remained significant even after adjustment of another well-studied biomarker, UACR.
Albuminuria is a well-known predictor of CVD [Citation33,Citation34], but the exact pathophysiological mechanism remains incompletely understood. It has been postulated that albuminuria is a late sign in the atherosclerotic process, and the leakage of albumin reflects a more generalized state of endothelial dysfunction. In a recent review, Mulè et al. presented post hoc analyses from trials in which reducing albuminuria led to a better cardiovascular outcome [Citation35]. Despite small numbers and heterogeneous data, a majority of the assessed trials supported this effect, also in patients without diabetes. However, most of these studies were not originally designed to test proteinuria as a primary treatment target and are therefore not well suited to give a definite answer as to whether reducing albuminuria per se causes better cardiovascular outcome [Citation4].
Several reports suggest that increased UOE is an earlier marker of disease than UAE. Elevated UOE levels have been found in women with preeclampsia, even in the presymptomatic latency period, before increments in UAE developed. In these women, plasma levels of orosomucoid also were higher, but the rise was found only late in the course of the disease, and thus the increase in UOE occurred independently of the plasma levels [Citation27]. Similar results were demonstrated by Christiansen et al. who found that increased UOE was an independent predictor for cardiovascular mortality, also in diabetic subjects with UAE < 20µg/min. These results support the hypothesis that UOE may be an early and more sensitive biomarker of inflammation and endothelial dysfunction [Citation18]. In our study, UOCR and UACR were only moderately correlated (Spearman’s r 0.43), arguing against overlapping pathophysiological processes.
Orosomucoid is under normal conditions present in the plasma as well in the urine in much lower concentration than albumin (orosomucoid in plasma 0.1-1.0 g/L vs albumin 35 g/l). However, in acute inflammation UOE is increased to levels equal to or higher than UAE [Citation27,Citation36–38]. Furthermore, several studies have demonstrated an association between UOCR and chronic inflammation in diseases such as psoriasis, rheumatoid arthritis, Crohn’s disease, chronic kidney disease and bladder cancer [Citation14,Citation15,Citation39–41]. The mechanisms for increased UOE is still poorly understood, but inflammation, alterations in glomerular filtration, including disturbances in the endothelial surface layer, as well as proximal tubular secretion, may be involved also for orosomucoid [Citation42]. Regardless of the mechanism, publications reporting associations between this urinary biomarker and subclinical and clinical endpoints are warranted to elucidate its clinical value and our results add to close this knowledge gap.
There is a well-known association between albuminuria and subclinical impairment of both systolic and diastolic function [Citation35], but the clinical applicability of UAE for both monitoring and guidance of treatment in heart failure is limited [Citation43]. Heart failure with preserved EF (HFpEF) has over the last decades received increasing attention, as approximately 50% of patients with heart failure have preserved EF. Recent physiologic studies have shown an association between microvascular endothelial inflammation and DD [Citation44,Citation45]. A correlation has been described between indices of diastolic function and endothelial function in patients with type 2 diabetes and hypertension [Citation23]. In a study involving 176 patients with chronic heart failure, urinary orosomucoid was identified by proteomics and bioinformatics as a novel heart failure biomarker, with a positive correlation to N-terminal pro-brain natriuretic peptide and New York Heart Association (NYHA) classification. The authors suggested that these results might be related to the inflammatory condition of chronic heart failure [Citation13].
In the present study, analysis showed an association between UOCR and DD for men, independent of other cardiovascular risk factors. On the contrary, there was no independent association between UACR and DD. The subgroup of our cohort that underwent echocardiography was older, had lower mean eGFR and higher levels of UOCR compared to the remaining cohort. Since both DD and decreasing eGFR are age-related, this may in part explain our findings. However, other explanations must be sought for the distinction between UOCR and UACR in this regard. We hypothesize that elevated UOCR and UACR represent different pathophysiologic processes, or different stages of similar processes, in relation to DD. In our study, echocardiographic measurements from the right ventricle were lacking, and therefore persons who fulfilled two of three instead of three of four echocardiographic indices, were diagnosed with DD. Therefore, DD may have been overestimated. On the other hand, this may have diluted our results, thereby strengthening our findings.
Since statistical interaction between sex and UOCR for the association with DD was not significant, apparent sex differences should be interpreted with cautions. ROC AUC for DD in men increased borderline significantly when adding UOCR to the model (p = .071). Further studies are needed to investigate whether UOCR may be a valuable clinical tool with regards to DD and HfpEF and whether there are differences between men and women.
Previous studies have shown that albuminuria is associated with both initiation of carotid arteriopathy and progression, also in the general nondiabetic population [Citation46,Citation47]. The independent associations between UOCR and UACR and the measurements of carotid arteriopathy in our study were of similar magnitude. It should be noted that the cut off for the upper level of IMT and TPA, as well as the use of the upper quartiles of UOCR and UACR, may not correspond to clinically relevant disease staging or biomarker cut-offs. For TPA, AUC of the ROC increased significantly when adding UOCR to the model for men, and borderline significance was found for IMT in women. Prospective studies are needed to examine whether UOCR is a more sensitive and earlier biomarker for carotid arteriopathy in the general population than UACR.
The main strengths of our study are the large number of well-characterized participants, the high attendance rate observed in the Tromsø Study, and the collection of three urine samples per person. Moreover, ultrasound examinations of the carotid and the heart were done with good accuracy [Citation28]. However, some limitations need to be addressed. The cross-sectional design limits conclusions about the temporal relationship between the biomarkers and the endpoints. Causal inferences can never be made from observational studies. Furthermore, although strongly associated with clinical disease, dependent variables in our study were surrogate endpoints only. Prospective studies, examining the associations between the biomarkers and clinical endpoints should be done.
In conclusion, we have shown an association between UOCR and well accepted subclinical markers of CVD. Although UOCR correlated with several traditional cardiovascular risk factors, this association was independent. Since no interaction between sex and the urinary biomarkers was found, apparent sex-differences should be interpreted with caution. Further research, including observational studies of the association between UOCR and clinical endpoints such as myocardial infarction, stroke and reduced kidney function, is needed to assess whether UOCR is useful in clinical situations.
Supplemental Material
Download MS Word (20.8 KB)Acknowledgments
EBM and HS were responsible for collecting the carotid- and echocardiography data. JBK organized the urinary analyses for orosomucoid. RMA and MDS performed the statistical analyses and drafted the article. All authors participated in critical revision of the manuscript and have read and approved the final version.
Disclosure statement
All authors declare no conflicts of interest with respect to the contents of this manuscript.
Additional information
Funding
References
- Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385(9963):148–171.
- Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88(2):451–487.
- Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol. 2008;19(3):427–432.
- Fried LF, Lewis J. Albuminuria is not an appropriate therapeutic target in patients with CKD: the con view. Clin J Am Soc Nephrol. 2015;10(6):1089–1093.
- Matsushita K, Coresh J, Sang Y, CKD Prognosis Consortium, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative Meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525.
- Fournier T, Medjoubi NN, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482(1-2):157–171.
- Varghese SA, Powell TB, Budisavljevic MN, et al. Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol. 2007;18(3):913–922.
- Salmon AH, Ferguson JK, Burford JL, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23(8):1339–1350.
- Padberg JS, Wiesinger A, di Marco GS, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–343.
- Fandino-Vaquero R, Fernandez-Trasancos A, Alvarez E, et al. Orosomucoid secretion levels by epicardial adipose tissue as possible indicator of endothelial dysfunction in diabetes mellitus or inflammation in coronary artery disease. Atherosclerosis. 2014;235(2):281–288.
- Kustan P, Szirmay B, Horvath-Szalai Z, et al. Urinary orosomucoid: validation of an automated immune turbidimetric test and its possible clinical use. Biochem Med (Zagreb). 2016;26(3):421–430.
- Christiansen MS, Blirup-Jensen S, Foged L, et al. A particle-enhanced turbidimetric immunoassay for quantitative determination of orosomucoid in urine: development, validation and reference values. Clin Chem Lab Med. 2004;42(10):1168–1177.
- Hou LN, Li F, Zeng QC, et al. Excretion of urinary orosomucoid 1 protein is elevated in patients with chronic heart failure. PloS One. 2014;9(9):e107550.
- Kustán P, Kőszegi T, Miseta A, et al. Urinary orosomucoid a potential marker of inflammation in psoriasis. Int J Med Sci. 2018;15(11):1113–1117.
- Park YJ, Yoo SA, Hwang D, et al. Identification of novel urinary biomarkers for assessing disease activity and prognosis of rheumatoid arthritis. Exp Mol Med. 2016;48:e211.
- Jiang H, Guan G, Zhang R, et al. Increased urinary excretion of orosomucoid is a risk predictor of diabetic nephropathy. Nephrology (Carlton, Vic. 2009;14(3):332–337.
- Christiansen MS, Hommel E, Magid E, et al. Orosomucoid in urine predicts cardiovascular and over-all mortality in patients with type II diabetes. Diabetologia. 2002;45(1):115–120.
- Christiansen MS, Hommel E, Magid E, et al. Orosomucoid in urine is a powerful predictor of cardiovascular mortality in normoalbuminuric patients with type 2 diabetes at five years of follow-up. Diabetologia. 2005;48(2):386–393.
- Nemeth B, Peter I, Boncz I, et al. Urinary orosomucoid: a new marker of cardiovascular risk in psoriatic patients? TCRM. 2019;ume 15:831–837.
- Lorenz MW, Bickel H, Bots ML, PROG-IMT Study Group, et al. Individual progression of carotid intima media thickness as a surrogate for vascular risk (PROG-IMT): rationale and design of a Meta-analysis project. Am Heart J. 2010;159(5):730–736 e732.
- Mathiesen EB, Johnsen SH, Wilsgaard T, et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke. 2011;42(4):972–978.
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271.
- Bedirian R, Neves MF, Oigman W, et al. Correlation between diastolic function and endothelial function in patients with type 2 diabetes and hypertension. Open Cardiovasc Med J. 2016;10:212–220.
- Jacobsen BK, Eggen AE, Mathiesen EB, et al. Cohort profile: the tromso study. Int J Epidemiol. 2012;41(4):961–967.
- Levey AS, Stevens LA, Schmid CH, for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Vittinghus E. Preanalytical handling of stored urine samples, and measurement of beta 2-microglobulin, orosomucoid, albumin, transferrin and immunoglobulin G in urine by enzyme-linked immunosorbent assays (ELISA). Scand J Clin Lab Invest. 1990;50(8):843–849.
- Kronborg CS, Allen J, Vittinghus E, et al. Pre-symptomatic increase in urine-orosomucoid excretion in pre-eclamptic women. Acta Obstet Gynecol Scand. 2007;86(8):930–937.
- Eggen AE, Mathiesen EB, Wilsgaard T, et al. The sixth survey of the tromso study (tromso 6) in 2007-08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand J Public Health. 2013;41(1):65–80.
- Nagueh SF, Smiseth OA, Appleton CP, Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the uropean society of echocardiography and the uropean association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360.
- Stensland-Bugge E, Bønaa KH, Joakimsen O. Reproducibility of ultrasonographically determined intima-media thickness is dependent on arterial wall thickness. The Tromsø Study. Stroke. 1997;28(10):1972–1980.
- Fosse E, Johnsen SH, Stensland-Bugge E, et al. Repeated visual and computer-assisted carotid plaque characterization in a longitudinal population-based ultrasound study: the Tromsø study. Ultrasound Med Biol. 2006;32(1):3–11.
- Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–1666.
- van der Velde M, Matsushita K, Coresh J, Chronic Kidney Disease Prognosis Consortium, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative Meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352.
- Solbu MD, Kronborg J, Jenssen TG, et al. Albuminuria, metabolic syndrome and the risk of mortality and cardiovascular events. Atherosclerosis. 2009;204(2):503–508.
- Mule G, Castiglia A, Cusumano C, et al. Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol. 2017;956:279–306.
- Magid E, Guldager H, Hesse D, et al. Monitoring urinary orosomucoid in acute inflammation: observations on urinary excretion of orosomucoid, albumin, alpha1-microglobulin, and IgG. Clin Chem. 2005;51(11):2052–2058.
- Kustan P, Horvath-Szalai Z, Muhl D. Nonconventional markers of sepsis. EJIFCC. 2017;28(2):122–133.
- Kustan P, Szirmay B, Horvath-Szalai Z, et al. Urinary orosomucoid: a novel, early biomarker of sepsis with promising diagnostic performance. Clin Chem Lab Med. 2017;55(2):299–307.
- Szirmay B, Tarnok A, Sarlos P, et al. Elevated urinary orosomucoid excretion as a novel biomarker in crohn’s disease. Eur J Clin Invest. 2018;49(3):e13054.
- Li F, Yu Z, Chen P, et al. The increased excretion of urinary orosomucoid 1 as a useful biomarker for bladder cancer. Am J Cancer Res. 2016;6(2):331–340.
- Jerebtsova M, Saraf SL, Soni S, et al. Urinary orosomucoid is associated with progressive chronic kidney disease stage in patients with sickle cell anemia. Am J Hematol. 2018;93(4):E107–E109.
- El-Beblawy NM, Andrawes NG, Ismail EA, et al. Serum and urinary orosomucoid in young patients with type 1 diabetes: a link between inflammation, microvascular complications, and subclinical atherosclerosis. Clin Appl Thromb Hemost. 2016;22(8):718–726.
- Valente MAE, Damman K, Dunselman PHJM, et al. Urinary proteins in heart failure. Prog Cardiovasc Dis. 2012;55(1):44–55.
- Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2017;376(9):897.
- Gladden JD, Chaanine AH, Redfield MM. Heart failure with preserved ejection fraction. Annu Rev Med. 2018;69:65–79.
- Furtner M, Kiechl S, Mair A, et al. Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J. 2005;26(3):279–287.
- Jørgensen L, Jenssen T, Johnsen SH, et al. Albuminuria as risk factor for initiation and progression of carotid atherosclerosis in non-diabetic persons: the tromsø study. Eur Heart J. 2007;28(3):363–369.
