Abstract
Objective. To study left ventricular (LV) function and blood pressure (BP) at a long-term follow-up in women after severe pre-eclampsia. Design. In this single-centre, cross-sectional study, 96 patients were eligible for inclusion. LV function was examined by transthoracic echocardiography including tissue Doppler echocardiography and speckle tracking. BP was measured at rest using repeated non-invasive techniques. Results. We compared 36 patients with early-onset and 33 patients with late-onset pre-eclampsia with 28 healthy controls. Mean age (40 ± 3 years) and median time since delivery (7 ± 2 years) were similar across the study groups. The patients had 18% higher systolic BP (139 ± 15 mmHg) and 24% higher diastolic BP (87 ± 19 mmHg) than controls (p < .01). Hypertension was present in 23 patients (33%), where the estimated LV mass was 16% higher (p = .05) than in controls. The LV ejection fraction was 19% lower in the early-onset group (51 ± 4%; p = .01) and 14% lower in the late-onset group (54 ± 6; p = .04) compared with controls. LV global longitudinal strain was 18% lower in the patient group (–17.7 ± 2.1%) compared with controls (p = .01). Indicative of a more restrictive filling pattern, the diastolic indices showed a lower e′ mean (p < .01) and subsequently higher E/e′ ratio (p < .01). There were no significant differences in BP, systolic or diastolic function indices between the patient groups. Conclusion. We found sustained hypertension, higher LV mass and reduced LV systolic and diastolic function 7 y after severe pre-eclampsia. Our findings emphasize the importance of early risk stratification and clinical counselling, and follow-up for such cases.
Introduction
Pre-eclampsia affects up to 7% of all pregnancies globally with a higher prevalence in low- to medium-income countries [Citation1,Citation2]. Risk factors include advanced maternal age, being overweight, nulliparity, previous pre-eclampsia, the use of assisted reproductive technology, genetics, hyperglycaemia and hypertension [Citation3]. Pre-eclampsia is associated with an increased risk of cardiovascular diseases (CVDs) such as hypertension, ischaemic heart disease, stroke, and venous thromboembolism later in life [Citation4]. Whether this risk of CVD predates and confounds pre-eclampsia, or is a result thereof is still unclear. Retrospective studies have reported higher rates of maternal death and risk of CVD in early- versus late-onset disease [Citation5]. There are different haemodynamic profiles in early- versus late-onset pre-eclampsia, with increased peripheral resistance and lower cardiac output observed in the early-onset form [Citation6–8]. Hypertensive pregnancies are associated with long-term impairment of vascular function, despite well-controlled blood pressure (BP) levels [Citation9]. Echocardiographic studies have reported left ventricular (LV) hypertrophy and LV diastolic dysfunction postpartum in women after pre-eclampsia [Citation10,Citation11]. Publications on systolic ventricular function in women with a history of pre-eclampsia report diverse findings. Data range from subclinical LV dysfunction in subgroups of women who suffered early-onset pre-eclampsia [Citation12], to normal subsequent echocardiography [Citation13]. Differences in study outcomes could be explained by the heterogeneity of the study populations with respect to the severity of pre-eclampsia and burden of CVD risk factors, the timing of follow-up examinations, and methodology applied across the different studies. Comprehensive echocardiography using tissue Doppler and 2 D speckle tracking echocardiography might detect subclinical myocardial alterations [Citation14]. Such sensitive modalities are useful to evaluate any signs of myocardial affection in hypertensive individuals [Citation15].
The aim of our study was to characterize LV function and hypertension at a long-term (7-y) follow-up in women after severe pre-eclampsia. We compared the women after early- versus late-onset pre-eclampsia with healthy controls. We hypothesized that these women would experience reduced LV function and sustained hypertension in the long-term follow-up.
Materials and methods
Design and study population
We performed a cross-sectional, single-centre study at Oslo University Hospital from October 2013 to January 2016. All women who delivered at the Department of Obstetrics and were diagnosed with severe pre-eclampsia in 2005–2010 (defined as the index pregnancy) were sent an open invitation letter and this was followed up by a telephone interview.
The inclusion criteria were a history of pre-eclampsia with severe hypertension. Exclusion criteria were a diagnosis of hypertension before the pre-eclamptic pregnancy, ongoing pregnancy, ongoing breastfeeding, ongoing assisted reproductive technology, neoplastic disease therapy and debilitating psychiatric illness at follow-up. Inclusion criteria for the control group were a previous normotensive pregnancy and giving birth at the Department of Obstetrics at Oslo University Hospital Rikshospitalet from 2008 to 2009. Exclusion criteria for the control group were CVD, previous hypertensive pregnancy disorders, ongoing pregnancy or breastfeeding.
We performed clinical registration and examination with echocardiography at a median of 7 y after the index pregnancy with severe pre-eclampsia and compared groups with early- versus late-onset disease with the controls. The study was carried out in accordance with the Declaration of Helsinki [Citation16]. It was approved by the Regional Committee for Medical and Health Research Ethics (REK Southeast, No. 2013-585 b), and the local institutional board at Oslo University Hospital. Written informed consent was obtained from all study participants.
Clinical characteristics
Clinical characteristics including weight, height, smoking habit, any medication is taken, comorbidity and cardiovascular events were registered. Registration of clinical variables in the pre-eclamptic index pregnancy was based on obstetrical and clinical charts. BP registrations from non-invasive measurements using automated oscillometric devices were collected retrospectively at multiple time points: through every trimester, at the time of pre-eclampsia diagnosis, before, during and after delivery, at discharge and on postpartum follow-up within 3 months. The BP measures before delivery are presented in . Blood samples were collected, processed and analysed according to the standard protocol at Oslo University Hospital.
Table 1. Maternal and neonatal characteristics at pregnancy with severe preeclampsia.
Blood pressure measurements
Each woman’s BP was measured using an automated oscillometric device (Dinamap ProCare 300, GE Healthcare, Amersham, UK). An appropriately sized pneumatic cuff was applied around the upper right arm; then three consecutive measurements were made of brachial arterial systolic (S)BP and diastolic (D)BP after the woman had rested for 10 min in a supine position.
Echocardiography
Transthoracic echocardiography was performed with the women in the left decubital position. Recordings from parasternal and apical views were obtained using a digital ultrasound scanner (Vivid E9 with 3.5 MHz phased array transducer [M5Sc], GE, Horten, Norway). Conventional greyscale cine loops, pulsed and continuous wave Doppler sonographic measures of blood flow velocities and tissue Doppler cine loops of LV were recorded. The rate of acquisition during tissue Doppler imaging was >100 frames per second (FPS) and during conventional two-dimensional (2 D) imaging >40 FPS. Three consecutive cine loops were recorded and stored for offline analysis utilizing the commercially available EchoPac, version 13.1 (GE).
Parameters of LV end-diastolic volume (LV EDV), end-systolic volume (LV ESV) and ejection fraction (EF) were calculated using Simpson’s modified biplane method, tracing endocardial contours in the apical four- and two-chamber views [Citation17]. LV mass (LVM) was calculated from LV dimensions obtained from parasternal M-mode registrations according to the Devereux formula [Citation18]. LVM is reported as indexed to the body surface area (BSA) in agreement with current recommendations [Citation17], applying the Mosteller formula to index BSA [Citation19].
Pulsed wave Doppler blood flow velocities were recorded at the mitral valve tip, and in the LV outflow tract (LVOT). Cardiac output (CO) was calculated from stroke volume (SV), determined by pulsed-wave Doppler recordings, and multiplied by heart rate (HR). The mitral peak early (E) and late (A) diastolic flow velocities were measured, and the E to A ratio and E deceleration time (DT) were calculated.
Pulsed tissue Doppler recordings were obtained by positioning a predefined sample volume of 5 mm at the basal septum and lateral wall by the junction with the mitral annulus in an apical four-chamber view. The parameters were measured on three consecutive heart cycles and averaged. Peak systolic velocity (s′) and peak diastolic early (e′) mitral annular tissue velocities were reported as the average of the septal and lateral annular values. The E/e′ ratio was then calculated as described [Citation20].
Strain depicts myocardial deformation and is defined as a fractional change of tissue length expressed in percentage. Two-dimensional speckle tracking was applied by echocardiography (2 D-STE) measuring strain in the three apical image planes to obtain LV global longitudinal strain (LVGLS). The commercially available automated function image technique was applied for strain measurements and LVGLS was calculated using an 18-segment model of the myocardium [Citation21]. Negative strain represents shortening of a myocardial segment, while positive values represent lengthening. Myocardial segments with unacceptable tracking quality were excluded. Strain measurements from eight (8%) participants had to be rejected because of poor image quality. All echocardiographic recordings and analyses were performed by one investigator (LG).
Echocardiographic recordings from 12 randomly selected study subjects were reanalysed by an independent and experienced investigator (AQ). Intraobserver repeatability was obtained from two measures of observer A (LG). Interobserver repeatability was obtained from two measures of observer A (LG) and two measures from observer B (AQ) for the interclass correlation coefficient (ICC). The intraobserver repeatability ICC for VTI was 0.95; the ICC for EF was 0.96, and the ICC for GLS was 0.97. The interobserver repeatability ICC for VTI was 0.89, for EF it was 0.85 and for GLS it was 0.86. Repeatability analysis was performed using IBM SPSS Statistics (v. 25; IBM Corp., Armonk, NY, USA).
Haemodynamics
Mean arterial pressure (MAP) was calculated from the SBP and DBP [Citation22]. The Systemic Vascular Resistance Index (SVRI) was calculated from the echocardiographic measurements of CO and MAP, and omitting mean right arterial pressure from the equation under conditions of normal right atrial filling properties [Citation23].
Data handling
According to statements from the International Society for the Study of Hypertension in Pregnancy, severe pre-eclampsia was classified as new-onset hypertension with SBP ≥160 mmHg or DBP ≥110 mmHg in combination with proteinuria defined as ≥30 mg/mmol albumin: creatinine spot urine sample or ≥1+ on a repeat dipstick test. Early-onset pre-eclampsia was defined as onset before 34 weeks of gestation and late-onset was defined as onset at/or after 34 weeks of gestation [Citation24].
Being overweight was defined as a body mass index (BMI) ≥25 kg·m−2, and obesity was defined as BMI ≥30 kg·m−2, according to the World Health Organization (WHO) classification [Citation25]. Hypertension and diabetes were defined based on the participant’s report of a physician’s diagnosis and use of antihypertensive and antidiabetic drugs, respectively. Any family history of CVD was defined as participants reporting CVD in first-degree relatives.
Statistical analysis
Normally distributed data are presented as the mean ± standard deviation. Non-normally distributed data are presented as the median and interquartile range. Categorical variables are presented as absolute values in percentages. Histograms and Q–Q plots were used to evaluate continuous values for normality. Comparison of between-group differences was analysed using Student’s t test or analysis of variance for normally distributed data. Between-group differences in categorical data were studied using Fisher’s exact test or the χ2 test, as appropriate. All tests were two-sided. The results were considered statistically significant at p < .05. Standard statistical analyses were performed with IBM SPSS Statistics (v. 25).
Results
Study population
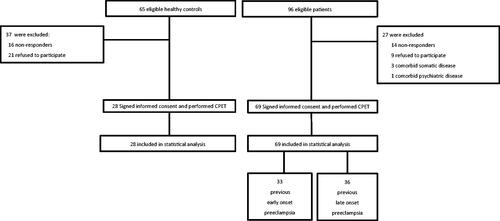
Ninety-six patients and 65 control subjects were identified and invited to participate in the study. Subsequently, 69 of the eligible patients with previous severe pre-eclampsia, and 28 healthy controls were included (). Thirty-three (48%) patients had early-onset and 36 (52%) patients had late-onset pre-eclampsia. Nine (13%) of the women in the early-onset group, and six (9%) women in the late-onset group had suffered more than one pre-eclamptic pregnancy at follow-up. Six (9%) patients in the early-onset group and four (6%) patients in the late-onset group had a history of one pre-eclamptic pregnancy before the index pregnancy, which was included in our retrospective clinical registration. Two (3%) patients in the early-onset group and one (1%) patient in the late-onset group suffered pre-eclampsia in later pregnancies until follow-up. Three (4%) women in the early-onset group had pre-eclampsia in all their three pregnancies until follow-up.
Background characteristics of the study population
Maternal and neonatal characteristics of the index pregnancy with severe pre-eclampsia are presented in . The groups were similar regarding age at follow-up, age at the index pregnancy and time since delivery. The high BPs recorded before delivery characterize the severity of pre-eclampsia.
Cardiovascular risk factors and hypertension
The maternal characteristics of the study participants at follow-up are presented in . BMI was 17% higher in the pre-eclamptic groups compared with controls (p < .01). Twenty-five patients (36%) were overweight, and 11 (16%) were obese based on their BMI. All control subjects were within the normal weight range defined by the WHO (see above).
Table 2. Maternal characteristics at follow-up after severe preeclampsia.
Hypertension was present in 23 patients (33%), of whom 14 (20%) had late-onset pre-eclampsia. Of these, three patients were diagnosed with moderate and two patients with severe hypertension at the follow-up visit. These patients were referred to further follow-up and treatment with their primary care physician. Women diagnosed with hypertension were treated with AT-II or ACE-inhibiting agents (n = 9), beta-blockers (n = 9), calcium-inhibiting agents (n = 4) or diuretics (n = 1). In patients with ongoing antihypertensive treatment, seven (11%) had moderate and one patient had severe hypertension. Two (3%) patients had suffered a myocardial infarction after the index pregnancy. Total cholesterol was normal in both pre-eclamptic groups (early-onset group 4.8 ± 0.8 mmol/l; late-onset group 4.7 ± 1.1 mmol/l; reference value 3.9–5.2 mmol/l). There were no significant differences in other cardiovascular risk factors such as diabetes mellitus, smoking, or family history of CVD between the early- and late-onset groups. Both groups had similar BP and CVD risk profiles in the index pregnancy, based on the clinical charts and a follow-up interview. At the 7-y follow-up, the patient groups had similar and significant CVD risk profiles.
Left ventricular function and haemodynamics at follow-up
The LV systolic function and haemodynamics at follow-up are presented in . Box plots of resting BP, LV EF and LV GLS are shown in . Both SBP and DBP were higher in both pre-eclamptic groups compared with controls (p < .01), with similar values between the early- and late-onset groups. In patients diagnosed with hypertension, the estimated LVM was 16% higher (p = .05) compared with normotensive patients. The LV EF was 19% lower in the early-onset group (p = .01) and 14% lower in the late-onset group (p = .04) compared with controls. Nine patients (13%) had an EF of 45%–50%. Of these, seven (10%) had poorly regulated hypertension. The LV GLS was 17% lower in the early-onset group (p = .01) and 19% lower in the late-onset group, compared with controls. The cardiac index was 20% lower in the early-onset group (p = .03) and 9% lower in the late-onset group (p = .07) compared with controls, and 7% lower in the early-onset group compared with the late-onset group (p = .09).
Figure 2. (A) Box plots showing systolic blood pressure in women after early-onset and late-onset preeclampsia, compared to controls. We found SBT to be significantly higher in both preeclamptic ic groups compared to controls. (B) Box plots showing left ventricular ejection fraction (LV EF) across the study groups. We found significantly lower LV EF in the early and late preeclamptic groups compared to controls. (C) Box plots showing left ventricular global longitudinal strain (LVGLS) in the study groups. We found LVGLS significantly lower in both early and late-onset preeclamptic groups compared to controls. We found no significant difference in SBT (p = .31, LVEF (p = .24), and LV GLS (p = .18) between the preeclamptic groups and controls (by ANOVA).
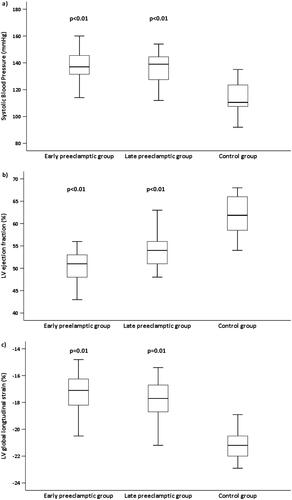
Table 3. Left ventricular systolic function and hemodynamics at follow-up after severe preeclampsia.
LV diastolic function at the follow-up after severe pre-eclampsia is shown in . There were no significant differences in diastolic function between the early- and late-onset groups. Both mitral inflow and early diastolic lengthening velocity were significantly lower in patients with moderate and severe hypertension (p < .05).
Table 4. Left ventricular diastolic function at follow-up after severe preeclampsia.
Number of pre-eclamptic pregnancies and CVD
At follow-up, the group of patients with more than one pre-eclamptic pregnancy (21%) had a higher proportion of hypertensive (n = 17) to normotensive patients (n = 4). They had 14% higher BMI, compared with women with one pre-eclamptic pregnancy, but a similar burden of other risk factors in terms of smoking, diabetes mellitus and family history of CVD. Patients with more than one pre-eclamptic pregnancy also showed higher SBP (9%) and LVM (12%), and lower LV GLS (6%). The diastolic indices in the patients with one versus more than one pre-eclamptic pregnancy were similar.
Discussion
This cross-sectional study aimed to characterize LV function and the incidence of hypertension on follow-up after severe pre-eclampsia. Seven years after pre-eclampsia, we found significantly lower EF and GLS indicating reduced LV systolic function. We also demonstrated lower LV diastolic function and an increased LVM compared with the healthy controls. One-third of the patients had hypertension, of whom eight (34%) had poorly regulated BP despite antihypertensive treatment. Women who suffered from pre-eclampsia also demonstrated significantly higher BMI values and familial predisposition for CVD.
Left ventricular systolic function
Our findings suggest that women with previous pre-eclampsia, both early and late-onset, have a reduced LV systolic function compared with healthy controls. Previous longitudinal studies have characterized reduced LV systolic function in pregnancies complicated by pre-eclampsia [Citation6,Citation26]. Melchiorre et al. demonstrated reduced myocardial performance persisting after pre-eclampsia under apparently normal loading conditions up to 2 y postpartum [Citation10]. They also found more severe myocardial impairment in early-onset compared with late-onset pre-eclampsia, associated with cardiac remodelling/hypertrophy and hypertension. Another study by Ghi et al., with a limited number of patients, also found reduced systolic function in Doppler indices and CO after severe pre-eclampsia [Citation27].
Importantly, longitudinal echocardiographic studies in normal pregnancies demonstrated by our group have previously found reduced LV EF, global myocardial strain, and contractility. The altered LV contractility and function were found to resolve within 6 months postpartum in healthy women [Citation28]. Findings of lower LV global strain in overweight pregnant women at term have been demonstrated by Zentner et al., associated with higher systemic vascular resistance and central arterial pressures [Citation29]. Melchiorre et al. showed that asymptomatic LV systolic and diastolic dysfunction, LV hypertrophy, and a prehypertension state persisted at 1-y postpartum, being more prevalent in early-onset compared with late-onset pre-eclampsia [Citation10]. Supporting our findings in the present study, subclinical LV systolic dysfunction may persist in long-term follow-up after pre-eclampsia. Clemmensen et al. reported reduced LV GLS in early-onset but not in late-onset groups at 12-y follow-up after severe pre-eclampsia [Citation12]. The GLS values in their study were similar to those in both the early- and late-onset groups in our study; the LV EF was within normal ranges and similar across their groups. In contrast to our study, they included fewer hypertensive patients. Their early-onset group had more hypertension and a seemingly higher cardiovascular risk profile compared with their late-onset group. The patient groups in our study were similar in these factors.
Myocardial strain is sensitive to increased afterload, and the relative degree of impairment of strain that is due to myocardial dysfunction compared with that caused by hypertension may be difficult to distinguish [Citation30]. We present data on the longitudinal strain, attributed to the endocardial layer of the myocardium. This precedes the alterations of circumferential strain, which is attributed to the mid-wall layer [Citation31].
The LV GLS has been shown to be abnormal in patients with normal EF, as well as in pre-hypertensive individuals [Citation32]. The prognostic value of EF in the range that is close to normal seems limited, while GLS does not show this limitation [Citation15]. This is of clinical importance as sensitive signs of end-organ damage on the myocardium—for instance from hypertension—should be subject to tighter follow-up and possibly more aggressive antihypertensive treatment [Citation30].
The hypertensive patients (n = 23) in our study showed lower LV GLS than the normotensive patients (p = .04). Those patients with established hypertensive treatment (n = 18) demonstrated a trend (p = .07) towards reduced GLS compared with normotensive patients. The hypertensive patients also demonstrated higher LVM compared with normotensive individuals (p = .03).
Left ventricular diastolic function
We found a statistically significant impaired diastolic function expressed by lower values in E wave velocity, diastolic peak early (e′) mitral annular tissue velocity, and the E/e′ ratio in both pre-eclamptic groups compared with controls. The E wave DT was also reduced in both pre-eclamptic groups. Despite the significant differences in these diastolic function parameters, none of the participants showed signs of increased filling pressures as measured by abnormal cut-off values E/e′ >14, E DT <130 ms, or E/A ratio >2 [Citation20]. Both the 2-y follow-up study of Melchiorre et al. [Citation10] and the 12-y follow-up study of Clemmensen et al. [Citation12] demonstrated persistent reductions in the parameters of diastolic function in women after early-onset pre-eclampsia, whereas diastolic function mostly recovered in women with late-onset pre-eclampsia.
Our findings on reduced diastolic echocardiographic indices in both early- and late-onset groups could be explained by similar occurrences of hypertension and LVM values. In cases of hypertension, a normal transmitral flow pattern indicates low risk for heart failure, independent of BP [Citation33]. Reportedly, the intermediate ranges of E/A ratio (0.6–1.5) are not useful to stratify their prognosis in hypertensive individuals [Citation34]. Tissue Doppler E/e′ ratio seems to be a better prognostic marker of cardiac risk in a well-treated hypertensive population [Citation35]. However, even with treatment intervention, changes in diastolic function are difficult to interpret clinically, as with cases of hypertensive heart disease [Citation36].
Persistent hypertension
Persistent hypertension on long-term follow-up after pre-eclampsia is consistently reported in clinical and epidemiological papers. We found persistent hypertension among the early- and late-onset groups at the long-term follow-up. The SBT values in the early- and late-onset groups were 20% and 17% higher, respectively. DBP-values in the early- and late-onset groups were 24% and 21% higher, respectively. Estimates of SVR, based on the measurements of CO and MAP, were over 30% higher in both pre-eclamptic groups compared with controls. This illustrates the significantly high afterload that LV must generate for cardiac output. The higher LVM (early-onset group 23% vs. controls, late-onset group 21% vs. controls) observed in both pre-eclamptic groups may reflect a persistently high afterload, leading to myocardial remodelling and altered geometry [Citation37]. It has been debated whether such reduced LV function and remodelling in the pathogenesis of hypertensive heart disease are a result of excessive afterload or are mediated through microvascular endothelial inflammation affecting the myocardium [Citation38,Citation39].
Increased LVM in cases of LV hypertrophy is considered a surrogate marker of CVD outcome, but it is still recognized as evidence of target organ damage in hypertension [Citation40]. However, repeated echocardiographic imaging evaluating LVM has been difficult to incorporate into clinical use in studies on hypertension. This is because of variability among measurements and low specificity as hypertrophy occurs in up to one-third of hypertensive patients, but can also be influenced by obesity and metabolic syndrome, among other aetiologies [Citation41].
Obesity and hypertensive heart disease
Obesity, independent of other comorbidities, can lead to subclinical changes in LV structure and function [Citation42]. Of note, our obese patients had 16% higher DBP (p = .04) and poorly regulated hypertension, as well as higher LVM and poorer LV functional indices. With the role that obesity possibly plays in relation to other pathological circumstances of increased SVR such as pre-eclampsia, our findings highlight the need for early and targeted intervention for achieving weight reduction among such patients.
Multiple pre-eclamptic pregnancies and CVD
Meta-analyses have shown that pre-eclampsia recurs in approximately 15% of subsequent pregnancies and is more common in those with early-onset disease. Recurrence of pre-eclampsia carries up to a threefold additional risk of developing hypertension and CVD later in life compared with a single pregnancy with pre-eclampsia [Citation43]. Pre-eclampsia recurred more frequently in our study, regardless of the initial severity. Most of our patients with more than one pre-eclamptic pregnancy had hypertension, and showed higher SBT and LVM, compared with patients with one pre-eclamptic pregnancy. These patients also had higher BMI. Our findings support the view that the recurrence of pre-eclampsia should be weighted even more strongly regarding the risk of future CVD [Citation43].
Interpretation and clinical implications
The mechanisms linking CVD and pre-eclampsia are thought to be multifactorial [Citation44]. Large studies have shown pre-eclampsia to be an independent risk factor for CVD after correcting for established risk markers such as hypertension [Citation4,Citation45]. A significant correlation between the recurrence of pre-eclampsia and the development of hypertension was also found in a recent systematic review [Citation46]. In addition, the time-specific association between pre-eclampsia and CVD is of relevance when interpreting study results. Hypertension has been demonstrated at 1 y after severe pre-eclampsia [Citation47]. Furthermore, studies with longer follow-up after pre-eclampsia still show a significant risk of CVD, and an even higher risk of CVD after recurrent pre-eclampsia [Citation48,Citation49]. Despite the strong associations between CVD and pre-eclampsia found in the literature, it remains unclear whether pre-eclamptic pregnancies actually induce metabolic and permanent CV changes or whether these women just have a stronger predisposition for CVD.
To our knowledge, our findings of persistent hypertension together with higher LVM, and reduced LV systolic and diastolic functions were detected earlier than in previous follow-up studies [Citation12]. The significant burden of CVD risk factors and a history of severe pre-eclampsia in our patients might help explain the early presentation of CVD in these premenopausal women.
Despite a significant subclinical reduction in LV function compared with controls, none of our patients had clinical signs of heart failure. Regardless, our findings are of concern especially as they were demonstrated in relatively young premenopausal women with hypertension persisting in one-third of women for 7 y after pre-eclampsia. Of note, none of the women had hypertension before their pre-eclamptic pregnancy. A significant number of our patients had poorly regulated hypertension despite treatment. Current European guidelines have already acknowledged hypertensive pregnancy disorder as a risk factor for future hypertension, stroke and ischaemic heart disease and recommend annual follow-up and lifestyle modifications to avoid future cardiovascular complications. In addition, the guidelines have recommended intense lifestyle modifications for patients in the prehypertensive category (SBP 130–140 mmHg and/or DBP 80–90 mmHg) [Citation50]. However, existing guidelines differ on when to start screening and follow-up, and on which health-care professionals should have the responsibility to establish a follow-up. At present, only half of the most commonly used guidelines advise cardiovascular risk assessment after pregnancy [Citation43].
In view of our findings and the existing literature, we argue that clinical follow-up and cardiovascular screening should be carried out soon after delivery. The women should be informed about the increased risk of future CVD after having had pre-eclampsia, and how to approach this risk. Lifestyle modifications, especially for overweight women, should be encouraged. Women with persisting hypertension after severe pre-eclampsia should be followed up and treated adequately. Moreover, in individuals with poorly regulated hypertension, echocardiography must be considered to evaluate the possibility of hypertensive heart disease, providing a basis for more aggressive treatment. Recurrent pre-eclampsia mandates even closer clinical follow-up.
Study limitations
Our cross-sectional study design and retrospective collection of data from index pregnancies had inherent limitations. Data were collected from a single centre with limited sample size. Some women in the pre-eclamptic groups declined participation, mostly explained by previous traumatic experiences from pregnancy and hospitalization. The absence of CVD risk factors in the control group excluded the opportunity of analysing the independent effects of pre-eclampsia on sustained hypertension and impaired LV function observed in the pre-eclamptic groups. That said, our controls had a risk profile and normal echocardiographic values similar to normal individuals in a large Nordic population [Citation51]. It would be preferable to have comprehensive longitudinal clinical and echocardiographic data before, during and after pregnancy to evaluate evolving CVD. The limited number of pre-eclamptic patients available prevented multivariable analysis. All parameters of systolic LV function are afterload dependent. As the patients with pre-eclampsia had higher BP than the controls, some of the reduction in LV function could be linked to the increased afterload. Increased afterload can be adjusted for by calculation of myocardial work and this should be implemented in future studies [Citation52]. Our patients had both first and repeat pregnancies before and after the index pregnancy. This is a confounding factor regarding the risk of CVD after pre-eclampsia from an index pregnancy. Because of our limited study sample, we could not conclude from our results what impact recurrent pre-eclampsia had on the risk of CVD. Further research is needed to establish a causative relationship and explanation of the pathogenesis of cardiovascular disease after pre-eclampsia.
We could not provide data on the excluded patients, including the non-responders, as we did not have approval from the Regional Committee for Medical and Health Research Ethics or the local institutional board at Oslo University Hospital to register data on patients not providing written informed consent. This may have biased our results.
Conclusions
Our study showed sustained hypertension, higher LV mass and reduced LV systolic and diastolic function 7 y after severe pre-eclampsia. These findings emphasize the importance of early risk stratification and clinical counselling, and long-term follow-up with adequate antihypertensive treatment for such women. Echocardiography to evaluate for hypertensive heart disease should be considered, especially for women with poorly regulated hypertension, emphasizing the need for more aggressive treatment.
Author contributions
MEE, EL and LG designed the study. LG was responsible for data collection, statistical analyses, and drafting the manuscript. HS contributed in writing the manuscript. AQ contributed to the interobserver analysis of echocardiographic data. The manuscript has been critically reviewed and approved by all authors.
Acknowledgements
We thank all those who took part in this study.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Macedo TCC, Montagna E, Trevisan CM, et al. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: a systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur J Obstet Gynecol Reprod Biol. 2020;248:177–186.
- Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7.
- Poon LS, Hyett JA, Kapur A, et al. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet. 2019;145(S1):1–33.
- Chappell LC, Cluver CA, Kingdom J, et al. Pre-eclampsia. Lancet. 2021;398(10297):341–354.
- Lisonkova S, Sabr Y, Mayer C, et al. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(4):771–781.
- Tyldum EV, Backe B, Stoylen A, et al. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand. 2012;91(5):566–573.
- Castleman JS, Ganapathy R, Taki F, et al. Echocardiographic structure and function in hypertensive disorders of pregnancy: a systematic review. Circ Cardiovasc Imaging. 2016;9(9):e004888.
- Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52(5):873–880.
- Collen AC, Hellgren M, Gustafsson H, et al. Cardiovascular and metabolic characteristics 40 years after hypertensive pregnancies: a long-term follow-up study of mothers. J Hypertens. 2013;31(4):758–765.
- Melchiorre K, Sutherland GR, Liberati M, et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–715.
- Vaddamani S, Keepanasseril A, Pillai AA, et al. Maternal cardiovascular dysfunction in women with early onset preeclampsia and late onset preeclampsia: a cross-sectional study. Pregnancy Hypertens. 2017;10:247–250.
- Clemmensen TS, Christensen M, Kronborg CJS, et al. Long-term follow-up of women with early onset pre-eclampsia shows subclinical impairment of the left ventricular function by two-dimensional speckle tracking echocardiography. Pregnancy Hypertens. 2018;14:9–14.
- Al-Nashi M, Eriksson MJ, Ostlund E, et al. Cardiac structure and function, and ventricular-arterial interaction 11 years following a pregnancy with preeclampsia. J Am Soc Hypertens. 2016;10(4):297–306.
- Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196–1207.
- Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680.
- World Medical A. World Medical Association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270.
- Roman MJ, Okin PM, Kizer JR, et al. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the strong heart study. J Hypertens. 2010;28(2):384–388.
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360.
- Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr. 2011;12(3):167–205.
- Klabunde RE. Cardiovascular physiology concepts. 2 ed. Philadelphia, PA: Lippincott Williams & Wilkins/Wolters Kluwer; 2012.
- Marino PLSK . The ICU book. 3 ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
- Tranquilli AL, Brown MA, Zeeman GG, et al. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens. 2013;3(1):44–47.
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World health Organ Tech Rep Ser. 2000;894:i-xii, 1–253.
- Paudel A, Tigen K, Yoldemir T, et al. The evaluation of ventricular functions by speckle tracking echocardiography in preeclamptic patients. Int J Cardiovasc Imaging. 2020;36(9):1689–1694.
- Ghi T, Degli Esposti D, Montaguti E, et al. Post-partum evaluation of maternal cardiac function after severe preeclampsia. J Matern Fetal Neonatal Med. 2014;27(7):696–701.
- Estensen ME, Beitnes JO, Grindheim G, et al. Altered maternal left ventricular contractility and function during normal pregnancy. Ultrasound Obstet Gynecol. 2013;41(6):659–666.
- Zentner D, du Plessis M, Brennecke S, et al. Cardiac function at term in human pregnancy. Pregnancy Hypertens. 2012;2(2):132–138.
- Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European association of cardiovascular imaging (EACVI) and the American society of echocardiography (ASE) dagger. Eur Heart J Cardiovasc Imaging. 2015;16(6):577–605.
- Galderisi M, Lomoriello VS, Santoro A, et al. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study. J Am Soc Echocardiogr. 2010;23(11):1190–1198.
- Di Bello V, Talini E, Dell'Omo G, et al. Early left ventricular mechanics abnormalities in prehypertension: a two-dimensional strain echocardiography study. Am J Hypertens. 2010;23(4):405–412.
- Wachtell K, Palmieri V, Gerdts E, et al. Prognostic significance of left ventricular diastolic dysfunction in patients with left ventricular hypertrophy and systemic hypertension (the LIFE study). Am J Cardiol. 2010;106(7):999–1005.
- Bella JN, Devereux RB, Roman MJ, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American indians (the strong heart study). Am J Cardiol. 2001;87(11):1260–1265.
- Sharp AS, Tapp RJ, Thom SA, et al. Tissue doppler E/E' ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010;31(6):747–752.
- Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369(9579):2079–2087.
- Santos M, Shah AM. Alterations in cardiac structure and function in hypertension. Curr Hypertens Rep. 2014;16(5):428.
- Hart CY, Meyer DM, Tazelaar HD, et al. Load versus humoral activation in the genesis of early hypertensive heart disease. Circulation. 2001;104(2):215–220.
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention. Detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572.
- Cuspidi C, Sala C, Negri F, et al. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J Hum Hypertens. 2012;26(6):343–349.
- Wong CY, O’Moore-Sullivan T, Leano R, et al. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–3087.
- Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105(16):1273–1278.
- Burton GJ, Redman CW, Roberts JM, et al. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
- Bellamy L, Casas JP, Hingorani AD, et al. Eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974.
- Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125(13):1642–1654.
- Benschop L, Duvekot JJ, Versmissen J, et al. Blood pressure profile 1 year after severe preeclampsia. Hypertension. 2018;71(3):491–498.
- Auger N, Fraser WD, Schnitzer M, et al. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017;103(3):235–243.
- Kessous R, Shoham-Vardi I, Pariente G, et al. Long-term maternal atherosclerotic morbidity in women with pre-eclampsia. Heart. 2015;101(6):442–446.
- Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314–340.
- Kou S, Caballero L, Dulgheru R, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15(6):680–690.
- Boe E, Russell K, Eek C, et al. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2015;16(11):1247–1255.