Abstract
Objectives. Atrial fibrillation is a common arrhythmia in patients with ischemic heart disease. This study aimed to determine the cumulative incidence of new-onset atrial fibrillation after percutaneous coronary intervention or coronary artery bypass grafting surgery during 30 days of follow-up. Design. This was a prospective multi-center cohort study on atrial fibrillation incidence following percutaneous coronary intervention or coronary artery bypass grafting for stable angina or non-ST-elevation acute coronary syndrome. Heart rhythm was monitored for 30 days postoperatively by in-hospital telemetry and handheld thumb ECG recordings after discharge were performed. The primary endpoint was the cumulative incidence of atrial fibrillation 30 days after the index procedure. Results. In-hospital atrial fibrillation occurred in 60/123 (49%) coronary artery bypass graft and 0/123 percutaneous coronary intervention patients (p < .001). The cumulative incidence of atrial fibrillation after 30 days was 56% (69/123) of patients undergoing coronary artery bypass grafting and 2% (3/123) of patients undergoing percutaneous coronary intervention (p < .001). CABG was a strong predictor for atrial fibrillation compared to PCI (OR 80.2, 95% CI 18.1–354.9, p < .001). Thromboembolic stroke occurred in-hospital in one coronary artery bypass graft patient unrelated to atrial fibrillation, and at 30 days in two additional patients, one in each group. There was no mortality. Conclusion. New-onset atrial fibrillation during 30 days of follow-up was rare after percutaneous coronary intervention but common after coronary artery bypass grafting. A prolonged uninterrupted heart rhythm monitoring strategy identified additional patients in both groups with new-onset atrial fibrillation after discharge.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice and is associated with an increased risk of stroke, heart failure, and death [Citation1,Citation2]. Postoperative AF after coronary artery bypass graft surgery (CABG) is associated with a 6-fold increased risk of future AF, reduced long-term survival, and increased risk of cardiovascular and cerebral mortality compared with CABG patients with no postoperative AF [Citation3]. In retrospective studies, patients with postoperative AF have a doubled long-term cardiovascular mortality compared with those who remain in sinus rhythm (SR), mainly explained by an increased risk of cardiac death and ischemic stroke [Citation3–7].
Over the last 30 years, the proportion of CABG for cardiac revascularization has decreased in favor of percutaneous coronary intervention (PCI), currently the most common revascularization strategy for coronary artery disease [Citation8]. In a post hoc analysis of the EXCEL trial, where CABG was compared to PCI for left main stem coronary disease, a post-procedural episode of AF was an independent predictor of stroke and death after three years [Citation9], importantly, AF after PCI was rare. However, systematic detection of post-procedure AF was not performed, and patients with preoperative AF were not systematically detected [Citation9]. Studies where the incidence of AF after CABG and PCI are compared, consist of non-systematic long-term postoperative AF detection [Citation10], or post-hoc analysis [Citation9,Citation11]. The Atrial Fibrillation after CABG and PCI (AFAF) study is a prospective observational study investigating the incidence of new-onset AF in patients undergoing either isolated CABG or PCI for stable/unstable angina or non-ST-elevation myocardial infarction (NSTEMI) during a 2-year follow-up period. Here we present a post-hoc interim analysis from the first 30-days of follow-up where we aimed to determine the cumulative incidence of new-onset post-operative/intervention AF in each group independently using an uninterrupted systematic post-procedure AF detection strategy.
Methods
Patients
Patients scheduled for isolated CABG or PCI were eligible for participation. Inclusion criteria were:
coronary intervention due to stable/unstable angina or NSTEMI
Exclusion criteria were:
history of AF
pacemaker or non-SR before coronary intervention
bleeding disorder contraindicating oral anticoagulation (OAC) therapy
cognitive impairment or communication problems leading to the inability to follow instructions and give informed consent
life expectancy <1 year
ongoing OAC therapy for any reason before the coronary intervention
for PCI patients, CABG surgery <3 months before PCI
for CABG patients, PCI <3 months before CABG surgery.
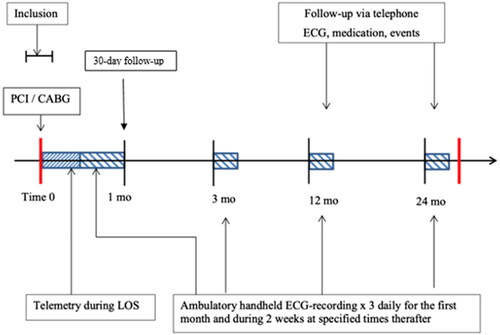
The study was conducted at three Swedish centers: Örebro University Hospital, Linköping University Hospital, and Danderyd Hospital, Stockholm. Patient enrollment started in May 2016 and ended in April 2019. The study followed the principles of the Declaration of Helsinki [Citation12]; approved by the regional ethics committee in Uppsala, Sweden (2015/413) and registered at ClinicalTrials.gov, NCT04307225. Signed informed consent was obtained from all participants. An external medical committee was appointed to evaluate adverse events and changes to the protocol. Eligible patients were consecutively asked to participate during their hospital stay for the coronary intervention with a span of 1 day before and 5 days after the index procedure (). The total follow-up period was 24 months. In this paper, we present results from the initial 30-days.
Arrhythmia monitoring and follow-up
New-onset AF was defined as an episode of >30 s of irregular R-R intervals and the absence of P waves. All CABG patients were monitored by telemetry for at least 4 days postoperatively and once terminated, a Zenicor II (Zenicor Medical Systems AB, Stockholm, Sweden) handheld thumb ECG device, was three times daily. PCI patients were monitored by telemetry during their hospital stay and once discharged with the Zenicor II device three times daily for the remainder of the 30-days (). The Zenicor II device provides one-lead 30-s ECG strip data to a web-based, password-protected, study-specific database. The device has been thoroughly validated as a reliable AF screening device [Citation13–15]. All recordings were monitored by study coordinators and, in cases of uncertain arrhythmia, by study cardiologists (EF, JE, TK, and JA). Any arrhythmia requiring intervention was reported to the patient and their local health care professional for further care. Thirty days after the index procedure, follow-up was conducted by on-site visit or telephone.
Antithrombotic therapy
All patients received antithrombotic therapy according to ESC guidelines [Citation16,Citation17]. After CABG, antiplatelet therapy with aspirin started on the day of surgery. In addition, patients were routinely anticoagulated with low-molecular-weight heparin (LMWH) for at least 5 days postoperatively. In the presence of acute coronary syndrome or drug-eluting stents (DES), dual antiplatelet therapy (DAPT) (i.e. aspirin and clopidogrel/prasugrel/ticagrelor) was optional postoperatively. After PCI, patients were routinely administered DAPT. For both groups, if the patient developed AF postoperatively, OAC with warfarin or DOAC (direct oral anticoagulants) was considered after estimating stroke risk using the CHA2DS2-VASc score, and individual bleeding risk assessment; With a CHA2DS2-VASc score ≥2, OAC was initiated unless contraindicated.
Statistical methods
Sample size was calculated by non-probability for a total of 2 years of follow-up, and as follows: Based on retrospective data and the assumption of an increased incidence by detection of silent AF, we estimated a cumulative incidence of AF of 35% in the CABG group and 15% in the PCI group. To obtain an estimated power of 90% and a significance level of .05, the total required number of participants was 194. To compensate for missing data and patient dropout during follow-up of 15%, the final required number of participants was 250. The 30-day results presented herein thus constitute a post-hoc analysis.
Categorical variables were analyzed using Fisher’s exact test, and the Mann–Whitney U test was used to analyze non-normally distributed variables. Probability values <0.05 were regarded as statistically significant. Data are mean ± standard deviation, % (n), or median (interquartile range). Predictors of AF were analyzed by univariate and multivariate logistic regression, with results presented as odds ratios (OR) with 95% confidence intervals (CIs). Variables selected were those of most clinical interest. All statistical analyses were performed using SPSS version 22 (IBM Corp, Armonk, NY, USA).
Results
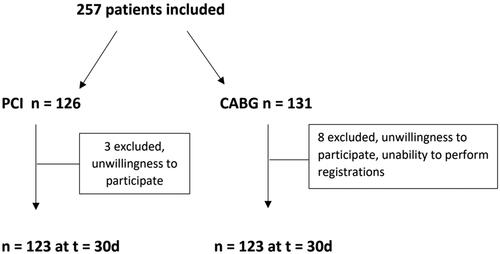
A total of 257 patients were included, 126 patients in the PCI group and 131 patients in the CABG group. At 30 days post-intervention, three patients were excluded from the PCI group and eight patients from the CABG group, providing 123 patients in each group as the final study population (). Missing data were limited to preoperative patient characteristic variables in the CABG group (Smoking status, alcohol consumption, congestive heart failure, previous MI, chronic obstructive pulmonary disease, obstructive sleep apnea, hypertension, diabetes mellitus, previous stroke, peripheral vascular disease) with n = 4 missing at the most, and preoperative alcohol consumption in the PCI group (n = 4).
Baseline characteristics
Patient characteristics are presented in . The gender distribution was 29% female in the PCI group and 9% female in the CABG group. There was no significant age difference. Diabetes mellitus (34 vs. 17%, p = .003) and heart failure (13 vs. 2%, p = .002) were more common in patients undergoing CABG than PCI, and CABG patients were more often affected by three-vessel disease (81 vs. 3%, p < .001). Chronic obstructive pulmonary disease (COPD) was more common in the PCI group and peripheral vascular disease was more common in the CABG group. Nearly all patients in the PCI group were discharged with DAPT (98%), whereas in the CABG group, 28/60 postoperative AF patients (46%) were discharged with OAC + aspirin (p < .001). At 30-days follow-up, 58 patients in the CABG group (84% of all with postoperative AF) were prescribed OAC.
Table 1. Preoperative patient characteristics.
New-onset AF
No patient in the PCI group experienced AF during hospitalization, and in the CABG group, 60/123 of patients (49%) had at least one episode of AF (p < .001). Most of the AF episodes were paroxysmal, and 6/123 patients (5%) were discharged with ongoing AF in the CABG group vs. 0/123 in the PCI group (p = .013) (). At 30-days, an additional, three patients in the PCI group and nine patients in the CABG group were diagnosed with new-onset AF, resulting in a cumulative incidence of AF of 3/123 patients (2%) after PCI and 69/123 (56%) after CABG (p < .001) (). CABG was a strong predictor for new-onset AF in both univariate (OR 51.1, 15.4–169.6, p < .001) and multivariate regression analysis (OR 80.2, 95% CI 18.1–354.9, p < .001) ().
Figure 3. Cumulative new-onset AF incidence. CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention.
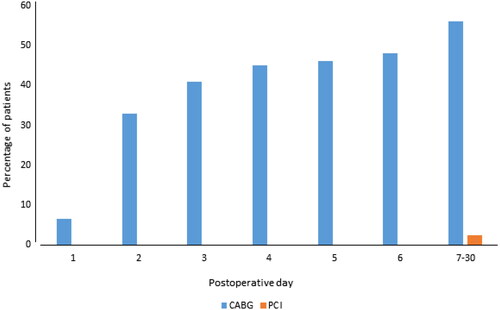
Table 2. In-hospital and discharge data.
Table 3. Predictors of new-onset atrial fibrillation 30 days postoperatively.
Complications
In-hospital rate of hemorrhagic incidents requiring transfusions, adjustment of medication, or surgical intervention was 2% in the PCI group and 8% in the CABG group (p = .046). At 30-days, bleeding events occurred in 1 CABG patient, who developed a hemorrhagic gastrointestinal ulcer during DAPT and NSAID treatment and was successfully managed non-surgically. No PCI patients experienced a bleeding event once discharged. Four patients in the CABG group developed clinically significant pericardial effusion, one of whom required surgical revision for late non-hemorrhagic cardiac tamponade.
Thromboembolic complications occurred in the hospital in one CABG patient (i.e. stroke) which was unrelated to AF, and none in the PCI group. At 30-day follow-up, one additional CABG patient who had developed AF and not yet initiated OAC, and one PCI patient who did not develop AF suffered thromboembolic stroke; There were no deaths ().
Table 4. 30-Day follow-up.
Discussion
In this 30-day follow-up of the AFAF study assessing the incidence of new-onset AF in patients undergoing PCI or CABG, the main findings were that AF is rare after PCI (2%) and common after CABG (56%), where CABG was a strong predictor for new-onset AF compared to PCI. In addition, new-onset AF occurred not only in conjunction with the index procedure but also following discharge, giving a cumulative increase in the incidence of new-onset AF over time. To our knowledge, this is the first prospective cohort trial comparing the incidence of new-onset AF after PCI and CABG.
New-onset AF has been reported to occur at a rate of 18–31% after CABG [Citation3,Citation4,Citation10] and 0.1–6% after PCI [Citation9,Citation18–21]. When interpreting these data, one must take into account several caveats. First, all studies reported the incidence of new-onset postoperative atrial fibrillation (POAF) based on in-hospital 12-lead ECG or telemetry, but information on AF is lacking beyond discharge. Second, the data are mainly retrospective and thus prone to detection bias. In the post-hoc analysis of the randomized EXCEL trial comparing PCI with CABG for left main stem coronary disease [Citation9], new-onset AF occurred in 18% of the CABG population and 0.1% of the PCI population. Notably, AF after CABG was an independent predictor of an increased risk of stroke, MI, and death at 3-year follow-up. Although similar differences between the groups, the incidence was significantly lower than in our study, which could be explained by several factors. The AF incidence was analyzed post hoc and therefore prone to confounding bias. CABG patients with no prior history of AF were to some extent administered preoperative amiodarone and/or beta blocker as AF prophylaxis. Moreover, follow-up data on arrhythmia were not systematically collected.
Other trials comparing PCI and CABG have found a consistent difference in the postoperative stroke rate. In the SYNTAX trial, comparing CABG with PCI for three-vessel disease and/or left main stem coronary artery disease, the stroke incidence at 1-year follow-up was 2.2% in the CABG group vs. 0.6% in the PCI group [Citation22]. In the Freedom trial, comparing CABG with PCI in diabetic patients with multi-vessel coronary artery disease, the stroke incidence at 5 years was 5.2% in the CABG group and 2.4% in the PCI group [Citation23]. Notably, differences in the incidence of postprocedural AF or in treatment with OAC were not recorded in these trials. About one-third of all AF is asymptomatic and often paroxysmal [Citation24], and the risk of stroke seems to be the same in silent and symptomatic AF [Citation25]. Overall, this implies that monitoring for AF beyond discharge is important for CABG patients.
Screening for silent AF using handheld ambulatory ECG recorders has proven to be an effective, noninvasive way of capturing episodes of AF and superior to 24-h Holter [Citation13,Citation26,Citation27]. Alternatively, continuous rhythm surveillance with implantable loop recorders can be performed, but this requires an additional invasive procedure. In the CABG population, this can be done safely as part of their surgery and provides rhythm and AF burden data [Citation28].
Data on the impact of new-onset AF after PCI are limited to retrospective data or post-hoc analyses; furthermore, they mainly investigate the in-hospital incidence of AF in an ST-elevation myocardial infarction (STEMI) population. New-onset AF incidence was 4.5–7%, and AF was a predictor of a worse cardiovascular outcome [Citation11,Citation18–21,Citation29].
The present results may shed some light on the etiology of postoperative AF. Both groups suffer from ischaemic heart disease requiring revascularization but with profoundly different results in the incidence of subsequent AF. Also, most participants were treated for stable angina and thus had no recent ischemic episode. Ischemia per se therefore seems less likely to be the inductive mechanism. However, the inflammatory responses after PCI and CABG differ [Citation30,Citation31], and inflammation is known to induce AF [Citation32–34]. Although attempts to reduce AF after cardiac surgery have been made by administering anti-inflammatory drugs, the effects have been moderate [Citation35]. The present study may indicate that further studies of inflammatory response after CABG and PCI are warranted.
Although postoperative AF has been shown to have a strong negative association with long-term cardiovascular survival after CABG, current guidelines recommend postoperative OAC treatment with a class IIa/b LOE B recommendation [Citation17,Citation36,Citation37]. As ischemic stroke constitutes a significant proportion of such events, underutilization of OAC treatment could theoretically explain the higher frequency of thromboembolic stroke after CABG. Retrospective data show conflicting results regarding OAC treatment in patients with postoperative AF [Citation38], and a current small scale RCT is expected to provide additional data for future recommendations [Citation39]. In all OAC treatment reduces the frequency of thromboembolic events in post-op AF CABG patients and seems safe in terms of bleeding complications. This mirrors our results where in-hospital bleeding events were rare in both groups and at 30-days almost non-existent. However, recommending or deterring routine use of OAC in these patients requires results from long term, large scale RCTs.
The major limitation of the present study is the non-randomized design leading to baseline differences and the absence of STEMI patients, The gender distribution is somewhat skewed in the CABG group, with 9% female participation, vs. 19% in the overall Swedish CABG population [Citation40]. The baseline differences reflect the guidelines for choice of revascularization method, with three-vessel disease and diabetes mellitus being more common among CABG patients. Thus any inferred causality between the groups cannot be made. Further, as the time of telemetry surveillance is generally shorter for the PCI group, concerns on whether early new-onset AF detection in the PCI group would be lacking, and non-comparable, could arise. However, previous data show that peak incidence and duration of new-onset AF is on postoperative day 2 and lasting 48 h [Citation3], thus it is unlikely that any episode of AF would not be captured by the thrice daily intermittent monitoring after discharge. Rather, a major strength of this study is the uninterrupted intermittent rhythm surveillance, yielding a cumulative increase in postoperative AF over time in both groups, which could explain the relatively higher prevalence of new-onset AF after CABG than previously reported.
Conclusion
Postoperative AF at 30-day follow-up occurs at a higher rate than previously reported after CABG and at a low rate after PCI. A prolonged uninterrupted heart rhythm monitoring strategy identified additional patients in both groups with new-onset AF after discharge from in-hospital care. Long-term follow-up with matched populations is needed to determine the prognostic impact of new-onset AF on cardiovascular morbidity and mortality as well as the predictive markers in each population.
Author contributions
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Acknowledgements
Thanks to the study nurses at all study sites for their valuable and arduous work in including patients, conducting follow-up, study logistics, and thumb-ECG interpretation:
Helen Hildingsson, Anna Mattson, Karin Johansson, and Margit Quist, Örebro University Hospital.
Liselotte Persson, Danderyd Hospital.
Maria Eriksson, Linköping University Hospital.
Thanks to Mikolaj Skibniewski M.D, and Emmanouil Charitakis M.D, Linköping University Hospital for their valuable assistance in patient follow-up and data management.
Thanks to Aron Sztaniszlav M.D Örebro University Hospital for aiding with the regression analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available upon request, please contact the corresponding author.
Additional information
Funding
References
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364. doi: 10.1016/s0002-9343(02)01236-6.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983.
- Ahlsson A, Fengsrud E, Bodin L, et al. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37(6):1353–1359. doi: 10.1016/j.ejcts.2009.12.033.
- Ahlsson A, Bodin L, Fengsrud E, et al. Patients with postoperative atrial fibrillation have a doubled cardiovascular mortality. Scand Cardiovasc J. 2009;43(5):330–336. doi: 10.1080/14017430802702291.
- Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742–748. doi: 10.1016/j.jacc.2003.11.023.
- Mariscalco G, Klersy C, Zanobini M, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118(16):1612–1618. doi: 10.1161/CIRCULATIONAHA.108.777789.
- Fengsrud E, Englund A, Ahlsson A. Pre- and postoperative atrial fibrillation in CABG patients have similar prognostic impact. Scand Cardiovasc J. 2017;51(1):21–27. doi: 10.1080/14017431.2016.1234065.
- SWEDEHART. SWEDEHART annual report 2021: SWEDEHEART Registry; 2021. Available from: https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh/1-swedeheart-annual-report-2021-english/viewdocument/3384
- Kosmidou I, Chen S, Kappetein AP, et al. New-onset atrial fibrillation after PCI or CABG for left main disease: the EXCEL trial. J Am Coll Cardiol. 2018;71(7):739–748. doi: 10.1016/j.jacc.2017.12.012.
- Potdar SP, Shales S, Baviskar M, et al. Incidence, predictors, and outcome for post-operative atrial fibrillation in Indian patients undergoing off-pump coronary artery bypass grafting–a prospective observational study. Indian J Thorac Cardiovasc Surg. 2022;38(4):366–374. doi: 10.1007/s12055-022-01358-7.
- Rene AG, Genereux P, Ezekowitz M, et al. Impact of atrial fibrillation in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention (from the HORIZONS-AMI [Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction] trial). Am J Cardiol. 2014;113(2):236–242. doi: 10.1016/j.amjcard.2013.09.016.
- Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
- Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J. 2009;43(3):163–168. doi: 10.1080/14017430802593435.
- Doliwa PS, Rosenqvist M, Frykman V. Paroxysmal atrial fibrillation with silent episodes: intermittent versus continuous monitoring. Scand Cardiovasc J. 2012;46(3):144–148. doi: 10.3109/14017431.2012.661873.
- Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, et al. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace. 2012;14(8):1112–1116. doi: 10.1093/europace/eur431.
- Kolh P, Windecker S, Alfonso F, et al. ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46(4):517–592.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88. doi: 10.1016/j.rec.2016.11.033.
- Chan W, Ajani AE, Clark DJ, et al. Impact of periprocedural atrial fibrillation on short-term clinical outcomes following percutaneous coronary intervention. Am J Cardiol. 2012;109(4):471–477. doi: 10.1016/j.amjcard.2011.10.004.
- Lopes RD, Elliott LE, White HD, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J. 2009;30(16):2019–2028. doi: 10.1093/eurheartj/ehp213.
- Mrdovic I, Savic L, Krljanac G, et al. Incidence, predictors, and 30-day outcomes of new-onset atrial fibrillation after primary percutaneous coronary intervention: insight into the RISK-PCI trial. Coron Artery Dis. 2012;23(1):1–8. doi: 10.1097/MCA.0b013e32834df552.
- Pilgrim T, Kalesan B, Zanchin T, et al. Impact of atrial fibrillation on clinical outcomes among patients with coronary artery disease undergoing revascularisation with drug-eluting stents. EuroIntervention. 2013;8(9):1061–1071. doi: 10.4244/EIJV8I9A163.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585.
- Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35(8):508–516. doi: 10.1093/eurheartj/eht491.
- Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45(9):2599–2605. doi: 10.1161/STROKEAHA.114.006070.
- Hendrikx T, Hornsten R, Rosenqvist M, et al. Screening for atrial fibrillation with baseline and intermittent ECG recording in an out-of-hospital population. BMC Cardiovasc Disord. 2013;13:41. doi: 10.1186/1471-2261-13-41.
- Hendrikx T, Rosenqvist M, Wester P, et al. Intermittent short ECG recording is more effective than 24-hour Holter ECG in detection of arrhythmias. BMC Cardiovasc Disord. 2014;14:41. doi: 10.1186/1471-2261-14-41.
- Sandgren E, Wickbom A, Kalm T, et al. The contribution of intermittent handheld electrocardiogram and continuous electrocardiogram monitoring with an implantable loop recorder to detect incident and recurrent atrial fibrillation during 1 year after coronary artery bypass graft surgery: a prospective cohort study. Heart Rhythm O2. 2021;2(3):247–54. doi: 10.1016/j.hroo.2021.05.001.
- Dal Zotto B, Barbieri L, Tumminello G, et al. New onset atrial fibrillation in STEMI patients: main prognostic factors and clinical outcome. Diagnostics. 2023;13(4):613. doi: 10.3390/diagnostics13040613.
- Ahlsson AJ, Bodin L, Lundblad OH, et al. Postoperative atrial fibrillation is not correlated to C-reactive protein. Ann Thorac Surg. 2007;83(4):1332–1337. doi: 10.1016/j.athoracsur.2006.11.047.
- Wu M, Gu X, Li X, et al. C-reactive protein and inflammatory cytokines during percutaneous coronary intervention. J Vasc Res. 2016;53(1–2):39–48. doi: 10.1159/000447558.
- Kinoshita T, Asai T, Takashima N, et al. Preoperative C-reactive protein and atrial fibrillation after off-pump coronary bypass surgery. Eur J Cardiothorac Surg. 2011;40(6):1298–1303.
- Patel P, Dokainish H, Tsai P, et al. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21(9):1064–1070. doi: 10.1111/j.1540-8167.2010.01774.x.
- Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011;17(10):556–563. doi: 10.1016/j.molmed.2011.05.007.
- Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312(10):1016–1023. doi: 10.1001/jama.2014.11026.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612.
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149(1):e1–e156.
- Butt JH, Xian Y, Peterson ED, et al. Long-term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3(5):417–424. doi: 10.1001/jamacardio.2018.0405.
- Chapin TW, Leedahl DD, Brown AB, et al. Comparison of anticoagulants for postoperative atrial fibrillation after coronary artery bypass grafting: a pilot study. J Cardiovasc Pharmacol Ther. 2020;25(6):523–530. doi: 10.1177/1074248420929483.
- Batra G, Ahlsson A, Lindahl B, et al. Atrial fibrillation in patients undergoing coronary artery surgery is associated with adverse outcome. Ups J Med Sci. 2019;124(1):70–77. doi: 10.1080/03009734.2018.1504148.