Abstract
Objectives. Temporary mechanical circulatory support (TMCS) has become a component in the therapeutic strategy for treatment of cardiogenic shock as a bridge-to-decision. TMCS can facilitate recovery of cardiopulmonary function, end-organ function, and potentially reduce the surgical risk of left ventricular assist device (LVAD) implantation. Despite the improvements of hemodynamics and end-organ function, post-LVAD operative morbidity might be increased in these high-risk patients. The aim of the study was to compare outcomes after Heartmate 3 (HM3) implantation in patients with and without TMCS prior to HM3 implant. Methods. In this retrospective cohort study of all HM3 patients in the period between November 2015 and October 2021, patients with and without prior TMCS were compared. Patients’ demographics, baseline clinical characteristics, laboratory tests, intraoperative variables, postoperative outcomes, and adverse events were collected from patient records. Results. The TMCS group showed an improvement in hemodynamics prior to LVAD implantation. Median TMCS duration was 19.5 (14–26) days. However, the TMCS group were more coagulopathic, had more wound infections, neurological complications, and more patients were on dialysis compared with patient without TMCS prior to HM3 implantation. Survival four years after HM3 implantation was 80 and 82% in the TMCS (N = 22) and non-TMCS group (N = 41), respectively. Conclusion. Patients on TMCS had an acceptable short and long-term survival and comparable to patients receiving HM3 without prior TMCS. However, they had a more complicated postoperative course.
Introduction
In patients suffering from cardiac arrest or acute refractory cardiogenic shock, temporary mechanical circulatory support (TMCS) has become an often employed component in the therapeutic strategy [Citation1–7]. Thus, TMCS is used with the aim of improving hemodynamics, and end-organ function in selected patients until ventricular recovery or either durable MCS implantation or transplantation [Citation1–3,Citation5–10].
Previous studies suggest that the hemodynamic improvement of patients on TMCS who are classified as an Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile 1, has hemodynamics comparable to profile 2 and 3 patients prior to durable MCS implantation [Citation1–3].
Despite the improvements of hemodynamics and end-organ function, post-left ventricular assist device (LVAD) operative morbidity in patients receiving TMCS remains high [Citation1,Citation3,Citation6,Citation7,Citation9,Citation11]. Moreover, the use of TMCS in this high-risk patient population is increasing while the number of LVAD implantations is not [Citation7,Citation12–14].
The aim of this study was to identify differences in short- and long-term clinical outcomes between patients who receive Heartmate 3 (HM3) with and without prior TMCS.
Materials and methods
The study was conducted in accordance with the principles of the Declaration of Helsinki and is approved by the Regional Ethical Review Board at the University of Copenhagen (R-21014768, approved 22 March 2021). Written consent was obtained.
Study design
This retrospective cohort study was based on all patients who received a HM3 between November 2015 and October 2021. Patients on TMCS prior to HM3 were compared with HM3 patients without prior TMCS. Types of TMCS included in this study were veno-arterial extracorporeal membrane oxygenation (V-A ECMO), percutaneous microaxial pumps (Impella CP and 5.0), and surgically implanted temporary central LVAD (Levitronix CentriMag). None of the patients were on intra-aortic balloon pump (IABP) prior to HM3. All patients on TMCS were fully neurological recovered prior to HM3.
Patients’ demographics, baseline clinical characteristics, laboratory tests, intraoperative variables, and postoperative outcomes were collected from our electronic patient records. Information regarding the vital status was attained by searching the Danish Central Civil Register.
Surgical technique
All HM3 procedures were performed through a full midline sternotomy. After systemic heparinization, cannulation for cardiopulmonary bypass (CPB) was performed via the ascending aorta and right atrium. For patients, who required concomitant persistent foramen ovale (PFO) closure a bicaval cannulation was performed. After conversion to CPB, TMCS was stopped and removed. Cardioplegia and aortic cross clamping was only used in patients requiring concomitant procedures (aortic valve replacement, PFO closure).
After implantation of the HM3, CPB was gradually weaned. Delayed chest closure was used in cases with hemodynamic instability or expected coagulopathy.
Definitions and outcomes
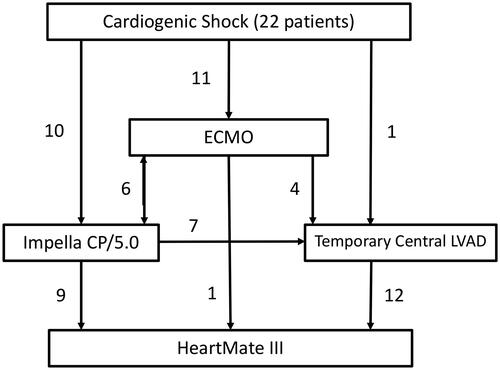
Preoperative hemodynamic values were examined with right heart catheterization (RHC) and Swan-Ganz catheterization (SGC) in the non-TMCS group prior to implantation and SGC was used in the TMCS group prior to HM3 implantation. Types and time on TMCS were noted ().
Figure 1. Mean days on ECMO, Impella CP/5.0, and Temporary Central LVAD (Levitronix CentriMag) where 5.2 (±2.8), 10.2 (±6.4), and 20 (±6.2) respectively. Median TMCS duration 19.5 (14–26) days.
Laboratory parameters was examined to evaluate the end-organ function before and after implantation of the HM3 as well as prior to TMCS and prior to HM3 while on TMCS. Analysis of interest was creatinine, estimated glomerular filtration rate (eGFR), international normalized ratio (INR), albumin, alanine aminotransferase (ALAT), bilirubin, and lactate dehydrogenase (LDH).
Postoperative bleeding was recorded, as well as the use of blood products. Postoperative bleeding was defined as bleeding measured in chest tubes until the next day at 06:00 am. Both groups were finished at the operating room around 02–04:00 pm the same day.
The adverse event definitions were based on the INTERMACS adverse event definition. Neurological deficits persisting more than 24 h both central and peripheral were recorded according to the consensus statement for definitions of adverse events [Citation15].
Outcomes include survival, patient status (alive on support, recovery, transplanted and death), 90 days mortality, operation for pump-complication, follow-up time, and time on HM3. Furthermore, adverse events such as GI bleeding, infections, neurological complications, and pump-thrombosis was noted. The cause of death was acquired from patient records if available. Routine follow-up was carried out by our Department of Cardiology.
Statistical analysis
The Shapiro–Wilk test was used to determine the normality of the continuous variables. Categorical data are presented with numbers (percentage).
Mean values were compared using the Student’s t-test. Median values were compared with Wilcoxon rank-sum test. Categorical values were compared with chi-squared test.
Survival analyses are illustrated by Kaplan–Meier (KM) plots and Cox regression model were used to assess the various covariates effect on survival. Data were censored on 4 January 2022. A p value of less than .05 was considered significant. Statistical analysis was performed by STATA MP 16.
Results
Baseline and characteristics
Sixty-three patients received a HM3. There were more males in both the TMCS and non-TMCS group. The TMCS group was younger, had more patients with ischemic etiology, and fifty percent were on dialysis prior to HM3 implantation (). The non-TMCS group had a more chronic etiology of heart failure, and more comorbidities ().
Table 1. Baseline characteristics prior to HM3 implantation.
TMCS configuration and end-organ function
Twenty-two patients were bridged with TMCS. Eleven patients were bridged with V-A ECMO. Six of the V-A ECMO patients received an Impella device (three with Impella CP and three with Impella 5.0) (four went from V-A ECMO to Impella and two were implanted with V-A ECMO and Impella simultaneously), and four were bridged to Levitronix CentriMag. Only one patient was bridge from V-A ECMO directly to HM3. Ten patients received an Impella device. Seven of the Impella patients were bridged to a temporary central LVAD (three patients with Impella CP and four with Impella 5.0), whereas nine were implanted with HM3 directly from Impella (three from Impella CP, five from Impella 5.0 and one who had both Impella CP and 5.0). One patient went from a temporary central LVAD directly to HM3 (post cardiotomy shock), and a total of twelve patients were on temporary central LVAD before implantation with HM3 (). Median TMCS duration was 19.5 days (14–26) days. However, mean number of days on V-A ECMO, Impella CP/5.0, and temporary central LVAD were 5.2 (±2.8), 10.2 (±6.4), and 20 (±6.2) respectively ().
Fifteen (68%) patients had cardiac arrest and 11 were placed on V-A ECMO during ongoing cardiopulmonary resuscitation. Ten of these patients experienced In Hospital Cardiac Arrest (IHCA). Eight of the IHCA patients were admitted due to myocardial infarction whilst two were admitted due to progression of heart failure. Five of the fifteen patients with cardiac arrest with cardiogenic shock experienced Out of Hospital Cardiac Arrest (OHCA). These patients were admitted to the hospital with ongoing cardiopulmonary resuscitation with an automated cardiopulmonary resuscitation (CPR) device. Furthermore, seven patients had cardiogenic shock without cardiac arrest prior to TMCS implantation (31.8%). Two of these patients experienced progression of their heart failure, four had a myocardial infarction, and one had a failure of weaning of CPB after elective surgery for mitral insufficiency.
During TMCS there was an improvement in ALAT, albumin, and bilirubin values prior to HM3 implantation, although not statistically significant (). There was a slight increase in creatinine during TMCS, but this was not statistically significant. LDH was significantly lower prior to implantation of HM3 (). Half of the patients were on dialysis prior to HM3 implantation in the TMCS group.
Table 2. Laboratory parameters pre-TMCS, pre-HM3 without TMCS, and pre-HM3 with TMCS.
Pre- and perioperative characteristics
TMCS group had satisfactory hemodynamics prior to HM3 implantation (). No difference was found regarding skin-to-skin time, extracorporeal circulation (ECC) time, other procedures during operation, bleeding, and transfusion of fresh frozen plasma (). The TMCS group received more blood and platelet transfusions ().
Table 3. Preoperative hemodynamics prior to HM3 implantation.
Table 4. Perioperative data.
Postoperative outcomes
The TMCS group had longer stay in the intensive care unit (ICU) after HM3 implantation, more hours on ventilator support, prolonged hospital length of stay (LOS) and delayed closure of sternum (). No patients required renal replacement therapy when discharged to home. The TMCS group had more wound infections, and overall, more neurological complications (). Event per patients-years (EPPY) of neurological complications was 0.057 in the non-TMCS group (41 patients;105 patient-years), 0.173 in TMCS group (22 patients;46 patient-years), and 0.093 in the whole population (63 patients;150 patient-years). Long-term complications are summarized in . Eleven patients died during the period of this study, and three of the non-TMCS patients and none of the TMCS patients died within 30 days post-LVAD implantation (). Eleven patients (27%) in the non-TMCS group and 8 (36%) in the TMCS group were transplanted. Only 1 (2%) patient in the non-TMCS group achieved recovery and the LVAD was explanted. Median (interquartile range (IQR)) follow-up time was 995 (267–1575) days in the non-TMCS group, and 831.5 (328–1103) days in the TMCS group. Furthermore, median (IQR) days on HM3 was 485 (210–1090) in the non-TMCS group, and 407 (244–898) in the TMCS group. There were no significant difference regarding, transplantation, recovery, follow-up time, and days on HM3. Multivariate cox proportional hazard model controlling for age, sex and TMCS did not show any statistically significant difference in survival regarding postoperative complications (Supplement 1). Lastly, KM plots censored for follow-up demonstrated satisfactory survival for both TMCS and non-TMCS group after four years with 80 and 82% respectively ().
Figure 2. Kaplan–Meier graph of survival censored for follow-up shows a four year survival of 80 and 82% for the TMCS and non-TMCS group.
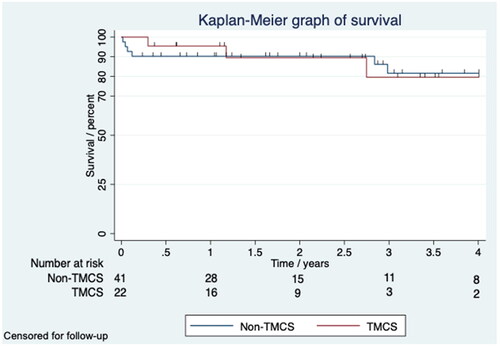
Table 5. Early postoperative outcomes and complications (<30 days).
Table 6. Adverse events after long-term follow-up (event per patient-years).
Discussion
The primary findings of our retrospective cohort study were that patients bridged with TMCS to HM3 had similar survival compared to non-bridged patients. However, the postoperative course was prolonged with more days in ICU and a longer stay in hospital. Patients in the TMCS group had increased risk of infection and neurological complications. Although half of the patients were on dialysis at the time of HM3 implantation none of the patient’s required dialysis at discharge.
In the MOMENTUM 3 trial the risk of stroke was 0.05 event/patients-years in the HM3 group [Citation16]. However, in this trial patients on TMCS were not included. We found a stroke rate of 0.057 EPPY in the non-TMCS group, but a significant higher rate (0.173 EPPY) in the TMCS group. In the ELEVATE study the two-year follow-up reported that 10.2% had experienced a stroke. In this study 12% had pre-implant MCS [Citation17]. The increased risk of stroke in the TMCS group after HM3 implantation may be multifactorial, but these patients had a more complex both pre and postoperative course, with a long complex rehabilitation process after HM3 implantation, as the patients were severely weakened after the time on TMCS.
Timing the implantation of an LVAD from TMCS is a complex and challenging decision. Shah et al. recommend TMCS duration of five to seven days to recover end-organ function, as prolonged support may increase the risk for adverse events [Citation3]. Uil et al. showed that TMCS support with a median time of five to six days could stabilize and reverse end-organ function [Citation18]. Yet, mortality was high in both studies [Citation3,Citation18]. Furthermore, Durinka et al. demonstrated that the use of V-A ECMO to stabilize patients prior to LVAD implantation had a survival to discharge rate of seventy-six percent if they were bridged to an LVAD within 14 days [Citation4]. Additionally, a retrospective study showed a median duration of 14 days of support with a survival of seventy percent after one year. Moreover, the Bridge-to-LVAD-group had an inferior outcome compared to nonbridging group which could be explained by the increased rate of right heart failure (RHF) after durable LVAD implantation, with up to 27% requiring an right ventricular assist device (RVAD) post implantation [Citation5]. RHF after LVAD implantation is associated with poor outcomes and high mortality [Citation5,Citation19], and the use of V-A ECMO at the time of durable LVAD implantation has been associated with an increased rate of RHF and postoperative morbidity [Citation10,Citation11]. Furthermore, in a small retrospective cohort study by Schibilsky et al. they described using V-A ECMO prior to HM2 implantation where the INTERMACS profile of these patients where stabilized from profile 1, to profile 2 and 3, with a mean duration of 7.5 ± 2.5 days. However, most of the patients in this group had a non-ischemic etiology of heart failure compared to our group of TMCS patients, and their patient population was younger [Citation20].The INTERMACS profiles were not design for including TMCS patients and downgrading a patient from INTERMACS 1 to 2 or 3 after implantation of a TMCS is not validated. In our study, median TMCS duration was 19.5 days. However, time on V-A ECMO was relatively short, on average five days. Our strategy was to wean all patients from ECMO to a temporary LVAD. The main reason was to better evaluate right heart function. Many patients on ECMO had an Impella CP placed for left ventricle venting. In cases where Impella CP was not sufficient after ECMO weaning and the neurological status was not fully recovered, we changed to central LVAD when the likelihood for HM3 was high. In cases where we hoped for cardiac recovery, we changed to an Impella 5.0, If recovery did not occur, a HM3 was implanted. Changes from Impella 5.0 to central LVAD was due to dysfunction of the Impella 5.0.
Saeed et al. demonstrated a median duration of five days on V-A ECMO prior to durable MCS implantation. However, 42% developed RHF requiring mechanical RV support [Citation7]. In our study only 5% required a temporary RVAD implantation. An explanation for this could be our weaning strategy.
This study demonstrates that TMCS provided satisfactory optimization of hemodynamics and with an end-organ function that did not worsen prior to durable LVAD implantation compared to the non-TMCS group. Yoshioka et al. and Lebreton et al. demonstrated that TMCS could restore end-organ function and hemodynamics before durable LVAD implantation [Citation5,Citation11]. However, they and Saeed et al. found that the TMCS group had inferior early and late outcomes compared to the non-TMCS group, with increased morbidity [Citation5,Citation7]. Furthermore, it has been shown that pre-operative and early worsening of renal function post implantation of durable LVAD has been associated with poor outcome and post-operative RHF [Citation19]. Our data support that renal replacement therapy should not be an exclusion criterion for durable LVAD therapy as 50% where on dialysis prior to HM3 implantation and no patients where on dialysis when discharged from hospital (). This is also supported in in Saeed et al. [Citation7]. Lastly, few patients in both groups had RHF post HM3 implantation (). Although, our results differ from some studies, we have demonstrated similar and acceptable survival in both groups (). A possible explanation for this is most likely our patient selection for durable LVAD implantation. Additionally, it is important to specify that the non-TMCS group had a more “chronic” profile with more comorbidities compared to the TMCS group. Also, the prerequisite for the TMCS group to improve hemodynamically prior to HM3 implantation could be the fact that they had a more “acute” profile with younger age and less comorbidities. Furthermore, a large portion of patients in the TMCS group where extracorporeal CPR (eCPR) patients. This could also be due to our national consensus on patient selection in regards to eCPR [Citation21]. Selection of the right patients at the right time is still a challenging part in this patient group.
Limitations
This study is a single-center retrospective cohort analysis with relatively few patients, which has its limitations. Additionally, there were no records of TMCS patients who were not selected for LVAD implantation. Thus, creating a selection bias which cannot be neglected.
Conclusion
Patients bridged on average 20 days with TMCS to implantation of a durable LVAD had short and long-term survival comparable to that of non-bridged patients, but TMCS patients had a more complicated postoperative course. Appropriately selected patients bridged with TMCS could be suitable candidates for a durable LVAD.
Author contributions
Imran Jamal Iversen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Finn Gustafsson: Investigation; Methodology; Writing—review & editing. Kasper Rossing: Investigation; Writing—review & editing. Peter Hasse Møller-Sørensen: Investigation; Methodology; Writing—review & editing. Peter Skov Olsen: Methodology; Writing—review & editing. Christian Holdflod Møller: Conceptualization; Data curation; Investigation; Methodology; Writing—review & editing.
Presentation
Data were presented as a poster at the EACTS MCS Summit in Berlin in 2022.
Supplemental Material
Download MS Word (14.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are available on request to the corresponding author.
Additional information
Funding
References
- Kurihara C, Kawabori M, Sugiura T, et al. Bridging to a long-term ventricular assist device with short-term mechanical circulatory support. Artif Organs. 2018;42(6):589–596. doi: 10.1111/aor.13112.
- Urban M, Siddique A, Moulton MM, et al. Can we expect improvements in outcomes with centrifugal vs axial flow left ventricular assist devices in patients transitioned from extracorporeal life support? J Card Surg. 2019;34(11):1228–1234. doi: 10.1111/jocs.14232.
- Shah P, Pagani FD, Desai SS, et al. Outcomes of patients receiving temporary circulatory support Before durable ventricular assist device. Ann Thorac Surg. 2017;103(1):106–112. doi: 10.1016/j.athoracsur.2016.06.002.
- Durinka JB, Bogar LJ, Hirose H, et al. End-Organ recovery Is key to success for extracorporeal membrane oxygenation as a bridge to implantable left ventricular assist device. Asaio J. 2014;60(2):189–192. doi: 10.1097/MAT.0000000000000043.
- Yoshioka D, Takayama H, Garan AR, et al. Bridge to durable left ventricular assist device for refractory cardiogenic shock. J Thorac Cardiovasc Surg. 2017;153(4):752 .e5–762.e5. doi: 10.1016/j.jtcvs.2016.10.085.
- Tsyganenko D, Gromann TW, Schoenrath F, et al. Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support. Eur J Cardiothorac Surg. 2019;55(4):773–779. doi: 10.1093/ejcts/ezy351.
- Saeed D, Potapov E, Loforte A, et al. Transition from temporary to durable circulatory support systems. J Am Coll Cardiol. 2020;76(25):2956–2964. doi: 10.1016/j.jacc.2020.10.036.
- Imamura T, Kinugawa K, Ono M, et al. Bridge-to-bridge left ventricular assist device implantation strategy vs. primary left ventricular assist device implantation strategy. Circ J. 2020;84(12):2198–2204. doi: 10.1253/circj.CJ-20-0840.
- Ton VK, Xie R, Hernandez-Montfort JA, et al. Short- and long-term adverse events in patients on temporary circulatory support before durable ventricular assist device: an IMACS registry analysis. J Heart Lung Transplant. 2020;39(4):342–352. doi: 10.1016/j.healun.2019.12.011.
- Marasco SF, Lo C, Murphy D, et al. Extracorporeal life support bridge to ventricular assist device: the double bridge strategy: thoughts and progress. Artif Organs. 2016;40(1):100–106. doi: 10.1111/aor.12496.
- Lebreton G, Pozzi M, Mastroianni C, et al. Extracorporeal life support as a bridge to bridge: a strategy to optimize ventricular assist device results. Eur J Cardiothorac Surg. 2015;48(5):785–791. doi: 10.1093/ejcts/ezu516.
- Aubin H, Petrov G, Dalyanoglu H, et al. Four-year experience of providing mobile extracorporeal life support to out-of-center patients within a suprainstitutional network—outcome of 160 consecutively treated patients. Resuscitation. 2017;121:151–157. doi: 10.1016/j.resuscitation.2017.08.237.
- Aubin H, Petrov G, Dalyanoglu H, et al. A suprainstitutional network for remote extracorporeal life support. JACC Heart Fail. 2016;4(9):698–708. doi: 10.1016/j.jchf.2016.03.018.
- Kormos RL, Cowger J, Pagani FD, et al. The society of thoracic surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114–126. doi: 10.1016/j.healun.2018.11.013.
- Kormos RL, Antonides CFJ, Goldstein DJ, et al. Updated definitions of adverse events for trials and registries of mechanical circulatory support: a consensus statement of the mechanical circulatory support academic research consortium. J Heart Lung Transplant. 2020;39(8):735–750. doi: 10.1016/j.healun.2020.03.010.
- Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-year outcomes in patients With fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. 2022;328(12):1233–1242. doi: 10.1001/jama.2022.16197.
- Zimpfer D, Gustafsson F, Potapov E, et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE Registry. Eur Heart J. 2020;41(39):3801–3809. https://doi.org/10.1093/eurheartj/ehaa639.
- den Uil CA, Akin S, Jewbali LS, et al. Short-term mechanical circulatory support as a bridge to durable left ventricular assist device implantation in refractory cardiogenic shock: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2017;52(1):14–25. doi: 10.1093/ejcts/ezx088.
- Takeda K, Naka Y, Yang JA, et al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant. 2014;33(2):141–148. doi: 10.1016/j.healun.2013.06.025.
- Schibilsky D, Haller C, Lange B, et al. Extracorporeal life support prior to left ventricular assist device implantation leads to improvement of the patients INTERMACS levels and outcome. Schäfer A, ed. PLoS One. 2017;12(3):e0174262. doi: 10.1371/journal.pone.0174262.
- Mørk SR, Stengaard C, Linde L, et al. Mechanical circulatory support for refractory out-of-hospital cardiac arrest: a danish nationwide multicenter study. Crit Care. 2021;25(1):174. doi: 10.1186/s13054-021-03606-5.
